Advertisements
Advertisements
प्रश्न
Give reason for the following:
The blue colour of aqueous copper sulphate fades when it is electrolysed using platinum electrodes.
उत्तर
The blue colour of copper ions fades due to the decrease in Cu+2 ions, and finally, the solution becomes colourless as soon as the Cu+2 ions are finished.
APPEARS IN
संबंधित प्रश्न
Name the following :
The process of coating of iron with zinc
Copy and complete the following table:
| Anode | Electrolyte | |
| Purification of copper |
Choose the correct answer from the options given below:
The electrolyte used for electroplating an article with silver is
A. Silver nitrate solution
B. Silver cyanide solution
C. Sodium argentocyanide solution
D. Nickel sulfate solution
Out of Cu and Ag, which is more active?
List out the main applications of electrolysis.
The following question relate to the electroplating of an article with silver.
What should be the nature of the anode?
The following questions are about electroplating of copper wire with silver.
(a) What ions must be present in the electrolyte?
(b) Of what substance must the anode be made up of?
(c) What will the cathode be made up of?
(d) Write the equation for the reaction which takes place at the cathode.
The electrolyte used for electroplating an article with silver is ______.
Which solution is preferred as electrolyte, CuSO4 or FeSO4?
Electroplating steel objects with silver involves a three-step process.
Step 1: A coating of copper is applied to the object.
Step 2: A coating of nickel is applied to the object.
Step 3: The coating of silver is applied to the object.
-
- A diagram of the apparatus used for step 1 is shown
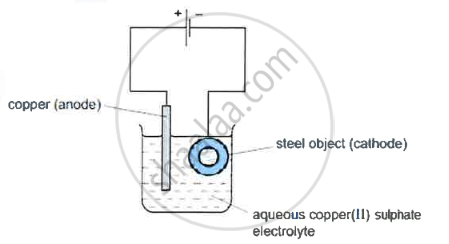
- The chemical process taking place on the surface of the object is \[\ce{Cu^2+(aq) + 2e- ->Cu(s)}\]
What is the observation seen on the surface of the object? - Explain why the concentration of copper ions in the electrolyte remains constant throughout step 1.
- The chemical process taking place on the surface of the object is \[\ce{Cu^2+(aq) + 2e- ->Cu(s)}\]
- A diagram of the apparatus used for step 1 is shown
- Give two changes which would be needed in order to coat nickel on to the object in step 2.
- Write down the reaction taking place at the positive electrode during step 3.
