Advertisements
Advertisements
प्रश्न
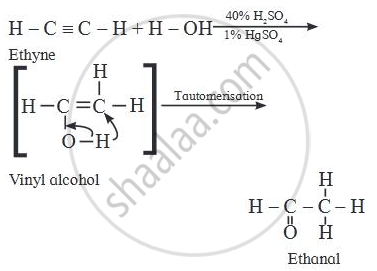
Identify the main product of the reaction:
\[\ce{H - C ≡ C - H + H - O ->[{40%} H2SO4][{1%} HgSO4]}\] _____.
उत्तर
Alkynes react with water in the presence of 40% sulphuric acid and 1% mercuric sulphate to form aldehydes or ketones i.e. carbonyl compounds.
Notes
In the textbook question is wrong.
APPEARS IN
संबंधित प्रश्न
Predict the possible product of the following reaction.
Chlorination of nitrobenzene
Predict the possible product of the following reaction.
sulphonation of chlorobenzene
Predict the possible product of the following reaction.
bromination of phenol
Identify A, B, C in the following reaction sequence:
\[\ce{CH3 - CH = CH2 ->[Br2/CCl4][room temperature] A ->[Zn] B ->[dil. alkaline][KMnO4] C}\]
Identify giving reason whether the following compound is aromatic or not.
Identify the compound (A) in the following reaction
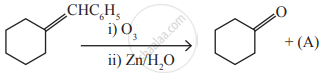
Which of the following compounds will not undergo Friedal – crafts reaction easily ? (NEET)
Which of the following can be used as the halide component for friedal - crafts reaction?
An alkane is obtained by decarboxylation of sodium propionate. Same alkane can be prepared by ______.
Which of the following is aliphatic saturated hydrocarbon?
Give IUPAC name for the following compound.
Ethyl isopropyl acetylene
Identify the compound A, B, C and D in the following series of reactions.

How is propyne prepared from an alkylene dihalide ?
Write the chemical equations for combustion of propane.
Write the structure of the following alkanes.
2, 3 – Dimethyl – 6 – (2 – methyl propyl) decane
Write the structure of the following alkanes.
5 – (1, 2 – Dimethyl propyl) – 2 – methylnonane
How will you prepare propane from a sodium salt of fatty acid?
Complete the following:
\[\begin{array}{cc}
\ce{CH2 - CH2 ->[Zn/C2H5OH]}\\
|\phantom{.......}|\phantom{..............}\\
\ce{Br}\phantom{.....}\ce{Br}\phantom{..............}
\end{array}\]
−Cl group is ____________.
Phenol on distillation with zinc dust gives ____________.
According to Huckel rule, a cyclic π molecular orbital formed by overlap of p orbitals must contain ____________ p electrons.
Dow's process is used for the synthesis of an aromatic compound (X). Identify X.
Conversion of hexane into benzene involves the reaction of ______.
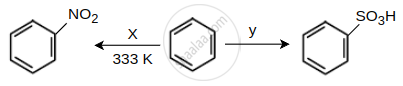
Identify the X and Y in the following reactions.
Read the following reaction and answer the questions given below.
\[\begin{array}{cc}
\phantom{..............................}\ce{CH3}\\
\phantom{...........................}|\\
\ce{CH3 - C = CH2 + HBr ->[Benzoyl][peroxide] H3C - CH - CH2Br}\\
|\phantom{....................................}\\
\ce{CH3}\phantom{.................................}
\end{array}\]
- Write the IUPAC name of the product.
- State the rule that governs the formation of this product.
Read the following reaction and answer the questions given below.
\[\begin{array}{cc}
\phantom{..............................}\ce{CH3}\\
\phantom{............................}|\\
\ce{CH3 - C = CH2 + HBr ->[benzoyl][peroxide] CH3 - CH - CH2Br}\\
|\phantom{....................................}\\
\ce{CH_3}\phantom{.................................}
\end{array}\]
- Write the IUPAC name of the product.
- State the rule that governs the formation of this product.
Read the following reaction and answer the questions given below.
\[\begin{array}{cc}
\phantom{..............................}\ce{CH3}\\
\phantom{...........................}|\\
\ce{CH3 - C = CH2 + HBr ->[benzoyl][peroxide] CH3 - CH - CH2Br}\\
|\phantom{....................................}\\
\ce{CH3}\phantom{.................................}
\end{array}\]
- Write the IUPAC name of the product.
- State the rule that governs the formation of this product.
