Advertisements
Advertisements
प्रश्न
Is it always necessary to use red light to get a photoelectric effect?
उत्तर
No, the broad wavelength and low energy of red light, photons do not have sufficient energy to pull an electron out of its orbital.
APPEARS IN
संबंधित प्रश्न
With the help of a circuit diagram describing an experiment to study the photoelectric effect.
What is the photoelectric effect? Define stopping potential and photoelectric work function.
The energy of a photon is 2 eV. Find its frequency and wavelength.
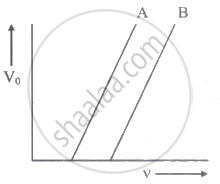
The following graph shows the stopping potential V0 versus frequency v for photoelectric emission from two metals A and B. The slope of each of the lines gives ______
The maximum velocity of the photoelectron emitted by the metal surface is 'v '. Charge and mass of the photoelectron is denoted by 'e' and 'm' respectively. The stopping potential in volt is ______.
Light of wavelength `lambda` strikes a photo-sensitive surface and electrons are ejected with kinetic energy E. If the kinetic energy is to be increased to 2E, the wavelength must be changed to `lambda'` where ____________.
Threshold wavelength for lithium metal is 6250 Å. For photoemission, the wavelength of the incident light must be ______.
Light of frequency 2 times the threshold frequency is incident on a photo sensitive material. If the frequency is made `1/3`rd and intensity is doubled then the photocurrent will ______.
When wavelength of incident radiation on the metal surface is reduced from 'λ1' to 'λ2', the kinetic energy of emitted photoelectrons is tripled. The work function of the metal is ______.
(h = Planck's constant, c =velocity of light)
Photoelectrons are emitted from a photosensitive surface for the light of wavelengths λ1 = 360 nm and λ2 = 600 nm. What is the ratio of work functions for lights of wavelength 'λ1' to 'λ2'?
Light of different frequencies, whose photons have energies 3 eV and 18 eV respectively, successively illuminate a metal of work function 2 eV. The ratio of the maximum speeds of the emitted electrons will be ______.
The photon of frequency vis incident on a metal surface whose threshold frequency is v0. The kinetic energy of the emitted photoelectrons will be ____________.
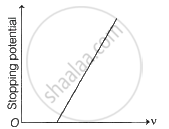
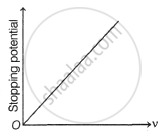
Following graphs show the variation of stopping potential corresponding to the frequency of incident radiation (F) for a given metal. The correct variation is shown in graph (v0 = Threshold frequency).
The photon of frequency vis incident on a metal surface whose threshold frequency is v0. The kinetic energy of the emitted photoelectrons will be ______.
When a photosensitive surface is irradiated by lights of wavelengths `lambda_1` and `lambda_2`, kinetic energies of emitted photoelectrons are E1 and E2 respectively. The work function of the photosensitive surface is ____________.
The ratio of slopes m1: ro2 of the lines given in the following graphs is, ______.
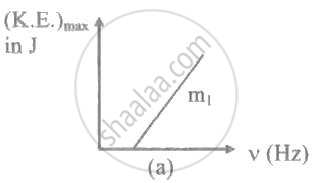
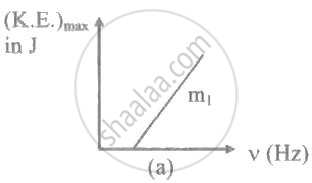
A light of frequency 'v' is incident on the metal surface whose threshold frequency is 'v0'. If v = v0, then [c = speed of light in medium] ____________.
In experiment of photoelectric effect, the stopping potential for incident yellow light of wavelength 5890 Å is 4 volt. If the yellow light is replaced by blue light of wavelength 4000 Å, the stopping potential is ____________.
The stopping potential in the context of photoelectric effect depends on the following property of incident electromagnetic radiation ______.
Photoelectrons are observed to just emit out of a material surface when the light of 620 nm falls on it with the intensity of 100 W m-2. If the light of wavelength 400 nm is incident on the same material with an intensity of 1 W m-2, what would be the minimum reverse potential needed to stop the outflow of the electrons?
The radiation emitted, when an electron jumps from n = 3 to n = 2 orbit is a hydrogen atom, falls on a metal to produce photoelectron. The electrons from the metal surface with maximum kinetic energy are made to move perpendicular to a magnetic field of `1/320`T in a radius of 10-3m. Find the 320 work function of metal:
The wavelength of light incident on a metal surface is reduced from 300 nm to 200 nm (both are less than threshold wavelength). What is the change in the stopping potential for photoelectrons emitted from the surface will be ______ V. (Take h = 6.6 × 10-34 J-s)
The maximum kinetic energy of the photoelectrons ejected will be ______ eV when the light of wavelength 350 nm is incident on a cesium surface. The work function of cesium = 1.9 eV.
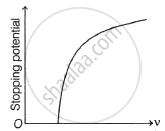
For a given photosensitive material and frequency (> threshold frequency) of incident radiation, the photoelectric current varies with the intensity of incident light as:
Light of wavelength λ, which is less than threshold wavelength is incident on a photosensitive material. If incident wavelength is decreased so that emitted photoelectrons are moving with same velocity, then stopping potential will ______.
The following graphs show the variation of stopping potential corresponding to the frequency of incident radiation (ν) for a given metal. The correct variation is shown in graph [ν0 = threshold frequency].
|
(A) |
(B) |
|
(C) |
(D) |
Photoelectric emission is observed from a metallic surface for frequencies ν1 and ν2 of the incident light rays (ν1 > ν2). If the ratio of the maximum value of the kinetic energy of the photoelectrons emitted in the first case to that in the second case is 2 : K, then the threshold frequency of the metallic surface is ______.
Explain the experimental set-up of photoelectric effect.