Advertisements
Advertisements
प्रश्न
How are polythene and neoprene prepared?
उत्तर
The antibiotic which is effective against a wide range of gram positive and gram negative bacteria known as broad spectrum antibiotic.
Preparating polyethene : The monomer used is ethene. It is an addition polymer. It is of two types.
\[\ce{n CH2 = CH2 ->[Δ] [-CH2 - CH2-]_n}\]
Preparating of Neoprne : The monomer used is chloroprene (2-chlorobuta-1,3-diene). It is superior to natural rubber and resistant to chemical action. It is used in the manufacture of chemical containers , conveyor belts, gaskets etc.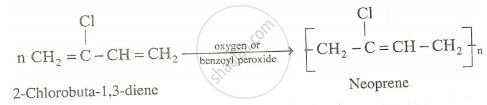
APPEARS IN
संबंधित प्रश्न
Write the molecular formula and structural formula of BHA and BHT.
What is the role of t-butyl peroxide in the polymerization of ethene?
Write the free radical mechanism for the polymerisation of ethene.
Write chemical reaction to prepare the Dextron polymer:
Dextron
Which of the following statements is not true about low-density polythene?
Out of chain growth polymerisation and step growth polymerisation, in which type will you place the following.

Match the polymer of column I with correct monomer of column II.
| Column I | Column II |
| (i) High density polythene | (a) Isoprene |
| (ii) Neoprene | (b) Tetrafluoroethene |
| (iii) Natural rubber | (c) Chloroprene |
| (iv) Teflon | (d) Acrylonitrile |
| (v) Acrilan | (e) Ethene |
Match the polymers given in Column I with their chemical names given in Column II.
| Column I | Column II |
| (i) Nylon 6 | (a) Polyvinyl chloride |
| (ii) PVC | (b) Polyacrylonitrile |
| (iii) Acrilan | (c) Polycaprolactum |
| (iv) Natural rubber | (d) Low-density polythene |
| (v) LDP | (e) cis-polyisoprene |
Assertion: Olefinic monomers undergo addition polymerisation.
Reason: Polymerisation of vinyl chloride is initiated by peroxides/ persulphates.
Assertion: Polytetrafluoroethene is used in making non-stick cookwares.
Reason: Fluorine has highest electronegativity.
Low density polythene and high density polythene, both are polymers of ethene but there is marked difference in their properties. Explain.
By which reaction ethene is obtained from ethyne?
Which one of the following can be used as monomer in a polymerisation reaction?
Which one of the following polymers are prepared by addition polymerisation?
Orlon fibres are made up of ______.
\[\ce{X + C + Cl2 ->[High temperature][of about 1000 K] Y + CO}\];
\[\ce{Y + 2H2O -> Z + 2HCl}\]
Compound Y is found in polymeric chain structure and is an electron-deficient Molecule. Y must be:
Which of the following statements about low density polythene is false?
