Advertisements
Advertisements
प्रश्न
Write one balanced equation to show Nuclear fission
Write any one balanced equation representing nuclear fission
उत्तर १
Nuclear Fission :
When a slow neutron strikes `""_92U^235` nucleus, it is absorbed by the nucleus and an isotope `U^236` is formed. But `U^236`, is highly unstable, is immediately broken into two fragments and emits neutrons and energy. This fission can be represented by the following equation
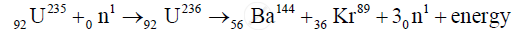
उत्तर २
Nuclear fission:
`""_0^1n + ""_92^235U -> ""_56^144Ba + ""_36^89Kr + 3 ""_0^1n`
APPEARS IN
संबंधित प्रश्न
Distinguish between nuclear fission and fusion. Show how in both these processes energy is released. Calculate the energy release in MeV in the deuterium-tritium fusion reaction :
`""_1^2H+_1^3H->_2^4He+n`
Using the data :
m(`""_1^2H`) = 2.014102 u
m(`""_1^3H`) = 3.016049 u
m(`""_2^4He`) = 4.002603 u
mn = 1.008665 u
1u = 931.5 MeV/c2
In a photon-electron collision ______.
(A) only total energy is conserved.
(B) only total momentum is conserved.
(C) both total energy and total momentum are conserved.
(D) both total momentum and total energy are not conserved
How long can an electric lamp of 100W be kept glowing by fusion of 2.0 kg of deuterium? Take the fusion reaction as
\[\ce{^2_1H + ^2_1H -> ^3_1He + n + 3.27 MeV}\]
Write notes on Nuclear fusion
Explain the processes of nuclear fission and nuclear fusion by using the plot of binding energy per nucleon (BE/A) versus the mass number A
In a nuclear reaction
`"_2^3He + _2^3He -> _2^4He +_1^1H +_1^1H + 12.86 Me V` though the number of nucleons is conserved on both sides of the reaction, yet the energy is released. How? Explain.
Show that the minimum energy needed to separate a proton from a nucleus with Zprotons and N neutrons is `ΔE = (M_(Z-1,N) + M_B - M_(Z,N))c^2`
where MZ,N = mass of an atom with Z protons and N neutrons in the nucleus and MB = mass of a hydrogen atom. This energy is known as proton-separation energy.
Consider the fusion in helium plasma. Find the temperature at which the average thermal energy 1.5 kT equals the Coulomb potential energy at 2 fm.
Calculate the Q-values of the following fusion reactions :-
(a) `""_1^2H + ""_1^2H → ""_1^3H + ""_1^1H`
(b) `""_1^2H + ""_1^2H → ""_2^3H + n`
(c) `""_1^2H + ""_1^3H → _2^4H + n`.
Atomic masses are `m(""_1^2H) = 2.014102 "u", m(""_1^3H) = 3.016049 "u", m(""_2^3He) = 3.016029 "u", m(""_2^4He) = 4.002603 "u".`
(Use Mass of proton mp = 1.007276 u, Mass of `""_1^1"H"` atom = 1.007825 u, Mass of neutron mn = 1.008665 u, Mass of electron = 0.0005486 u ≈ 511 keV/c2,1 u = 931 MeV/c2.)
Briefly explain the elementary particles present in nature.
