Advertisements
Advertisements
Questions
Write one balanced equation to show Nuclear fission
Write any one balanced equation representing nuclear fission
Solution 1
Nuclear Fission :
When a slow neutron strikes `""_92U^235` nucleus, it is absorbed by the nucleus and an isotope `U^236` is formed. But `U^236`, is highly unstable, is immediately broken into two fragments and emits neutrons and energy. This fission can be represented by the following equation
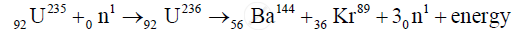
Solution 2
Nuclear fission:
`""_0^1n + ""_92^235U -> ""_56^144Ba + ""_36^89Kr + 3 ""_0^1n`
APPEARS IN
RELATED QUESTIONS
Calculate the energy in fusion reaction:
`""_1^2H+_1^2H->_2^3He+n`, where BE of `""_1^2H`23He=7.73MeV" data-mce-style="position: relative;">=2.2323He=7.73MeV MeV and of `""_2^3He=7.73 MeV`
How long can an electric lamp of 100W be kept glowing by fusion of 2.0 kg of deuterium? Take the fusion reaction as
\[\ce{^2_1H + ^2_1H -> ^3_1He + n + 3.27 MeV}\]
Free 238U nuclei kept in a train emit alpha particles. When the train is stationary and a uranium nucleus decays, a passenger measures that the separation between the alpha particle and the recoiling nucleus becomes x in time t after the decay. If a decay takes place when the train is moving at a uniform speed v, the distance between the alpha particle and the recoiling nucleus at a time t after the decay, as measured by the passenger will be
During a nuclear fission reaction,
Show that the minimum energy needed to separate a proton from a nucleus with Zprotons and N neutrons is `ΔE = (M_(Z-1,N) + M_B - M_(Z,N))c^2`
where MZ,N = mass of an atom with Z protons and N neutrons in the nucleus and MB = mass of a hydrogen atom. This energy is known as proton-separation energy.
Consider the fusion in helium plasma. Find the temperature at which the average thermal energy 1.5 kT equals the Coulomb potential energy at 2 fm.
Calculate the Q-values of the following fusion reactions :-
(a) `""_1^2H + ""_1^2H → ""_1^3H + ""_1^1H`
(b) `""_1^2H + ""_1^2H → ""_2^3H + n`
(c) `""_1^2H + ""_1^3H → _2^4H + n`.
Atomic masses are `m(""_1^2H) = 2.014102 "u", m(""_1^3H) = 3.016049 "u", m(""_2^3He) = 3.016029 "u", m(""_2^4He) = 4.002603 "u".`
(Use Mass of proton mp = 1.007276 u, Mass of `""_1^1"H"` atom = 1.007825 u, Mass of neutron mn = 1.008665 u, Mass of electron = 0.0005486 u ≈ 511 keV/c2,1 u = 931 MeV/c2.)
Explain in detail the four fundamental forces in nature.
Briefly explain the elementary particles present in nature.
The curve of binding energy per nucleon as a function of atomic mass number has a sharp peak for helium nucleus. This implies that helium nucleus is ______.
