Advertisements
Advertisements
प्रश्न
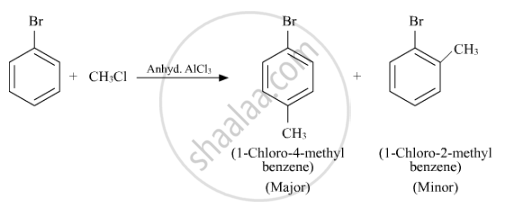
Write the structure of the major product in each of the following reaction :
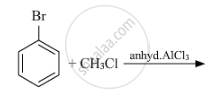
उत्तर
संबंधित प्रश्न
Write the structure of an isomer of compound C4H9Br which is most reactive towards SN1 reaction
Out of 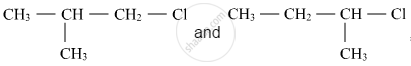 , which is more reactive towards SN1 reaction and why?
, which is more reactive towards SN1 reaction and why?
Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH(Br)CH2CH3 + NaOH ->[water]}\]
Arrange the compounds of the following set in order of reactivity towards SN2 displacement:
1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 2-Bromo-3-methylbutane
Out of C6H5CH2Cl and C6H5CHClC6H5, which is more easily hydrolysed by aqueous KOH.
Halogenation of alkanes is ____________.
Which of the following alkyl halides will undergo SN1 reaction most readily?
Which of the following statements are correct about the kinetics of this reaction?
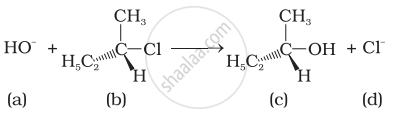
(i) The rate of reaction depends on the concentration of only (b).
(ii) The rate of reaction depends on concentration of both (a) and (b).
(iii) Molecularity of reaction is one.
(iv) Molecularity of reaction is two.
Convert bromoethane to propanamine.
Which alkyl halide from the following pair would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
\[\begin{array}{cc}\ce{CH3CHCH2CH2Br}\\|\phantom{.........}\\\ce{CH3}\phantom{......}\end{array}\] or \[\begin{array}{cc}\ce{CH3CH2CHCH2Br}\\\phantom{}|\\\phantom{...}\ce{CH3}\end{array}\]
