Advertisements
Advertisements
प्रश्न
Write the type of isomerism exhibited by [Co(NH3)5(NO2)]2+ and [Co(NH3)5ONO]2+ pair of complex ion.
उत्तर
The type of isomerism exhibited is linkage isomerism.
संबंधित प्रश्न
Out of 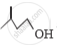 and
and 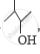 , which one is optically active and why ?
, which one is optically active and why ?
Answer the following question.
Draw isomers of the following
Ru(NH3)4Cl2
Answer the following question.
Draw geometric isomers and enantiomers of the following complex.
[Pt(en)2ClBr]2⊕
Draw optical isomers of [Co(en)3]3+.
Which one of the following complexes is not expected to exhibit isomerism?
What is linkage isomerism? Explain with an example.
The term anomers of glucose refer to ____________.
Which would exhibit coordination isomerism?
The number of geometrical isomers of [CrCl2(en)2]+ is ____________.
Consider the two complexes given below:
\[\ce{\underset{(I)}{[Co(NH3)5SO4]Br}}\] and \[\ce{\underset{(II)}{[Co(NH3)5Br]SO4}}\]
I and II are ____________ isomers.
____________ isomers are formed when the ligand has two different donor atoms.
The complex ions [Co(H2O)5(ONO)]2+ and [Co(H2O)5NO2]2+ are ____________.
\[\ce{IUPAC}\] name of \[\ce{[Pt(NH3)2 Cl(NO2)]}\] is ______.
The relationship between compound (i) and (ii) is
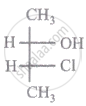 |
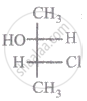 |
| (i) | (ii) |
Geometrical isomerism is not shown by
Which of the following does not show optical isomerism?
Complex [COCl2(en)2]+ can
Which of the following compound show optical isomerism?
Which of the following shows maximum number of isomers?
Indicate the type of isomerism exhibited by the following complex and draw the structures for this isomer:
\[\ce{[Pt(NH3)(H2O)Cl2]}\]
Explain the geometrical isomerism of the octahedral complex of the type [M(AA)2B2]n± with a suitable example.
Draw the structure of trans isomers of Pt(NH3)2Cl2.
Name the type of isomerism exhibited by the following pair of compound:
\[\ce{[Co(NH3)5 [ONO]Cl2 and [Co(NH3)5(NO2)]Cl2}\]
Name the type of isomerism exhibited by the following pair of compound:
\[\ce{[Cr(H2O)5Cl]Cl2H2O and [Cr(H2O)4Cl2]Cl {.} 2H2O}\]
Give a chemical test to show that \[\ce{[Co(NH3)5Cl]SO4}\] and \[\ce{[Co(NH3)5SO4]CI}\] are ionisation isomers.
Which one of the following complex ions has geometrical isomers?
