Diamonds have fascinated people throughout history for their beauty, rarity, and unique properties. Once renowned for the famous 'Kohinoor' diamond, India has been a prominent source of these precious gems, particularly in regions like Golconda and Panna. Diamonds are also found globally, in places such as South Africa, Brazil, and Russia. Their remarkable characteristics arise from their unique atomic structure, where each carbon atom is covalently bonded to four others in a three-dimensional arrangement, making diamonds incredibly hard.
Diamond
- Diamonds are the hardest natural substance, known for their exceptional durability and brilliance.
- The density of diamond is 3.5 g/cm³, reflecting its compact atomic structure.
- Diamonds have a very high melting point of 3500°C, making them resistant to extreme heat.
- When heated to 800°C in the presence of oxygen, diamonds release carbon dioxide without forming any other products.
- Diamonds do not dissolve in any solvent, showcasing their chemical stability.
- Acids and bases have no effect on diamonds, further highlighting their inert nature.
- Diamonds are poor conductors of electricity because they lack free electrons.
- These properties make diamonds valuable for industrial applications and prized as gemstones.
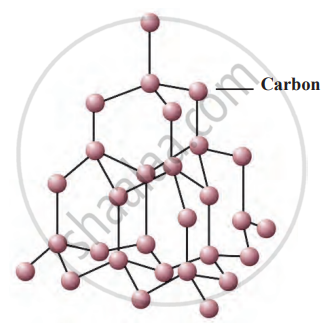
Structure of carbon atoms in diamond
