Advertisements
Advertisements
प्रश्न
Arrange the set of compounds in order of increasing boiling points.
1-Chloropropane, Isopropyl chloride, 1-Chlorobutane.
उत्तर
Molecules with the same mass have different boiling points based on their size. Branched compounds have a lower boiling point than straight-chain compounds due to their smaller surface area and compact structure. The order of boiling points is:
Isopropyl chloride < 1-Chloropropane < 1-Chlorobutane
APPEARS IN
संबंधित प्रश्न
Why dextro and laevorotatory isomers of Butan-2-ol are difficult to separate by fractional distillation?
Which of the following is liquid at room temperature (b.p. is shown against it)?
Which of the following possesses the highest melting point?
Mg reacts with RBr best in ____________.
Arrange the following compounds in the increasing order of their densities.
(a)
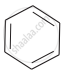
(b)
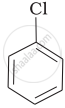
(c)
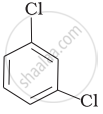
(d)
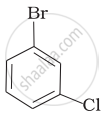
Arrange the following compounds in increasing order of their boiling points.
(a) \[\begin{array}{cc}
\ce{CH3}\phantom{.................}\\
\backslash\phantom{.............}\\
\ce{CH - CH2Br}\\
/\phantom{.............}\\
\ce{CH3}\phantom{.................}
\end{array}\]
(b) \[\ce{CH3CH2CH2CH2Br}\]
(c) \[\begin{array}{cc}
\phantom{...}\ce{CH3}\\
\phantom{}|\\
\ce{H3C - C - CH3}\\
\phantom{}|\\
\phantom{..}\ce{Br}
\end{array}\]
Which is the correct increasing order of boiling points of the following compounds?
1-Iodobutane, 1-Bromobutane, 1-Chlorobutane, Butane
Which is the correct increasing order of boiling points of the following compounds?
1-Bromoethane, 1-Bromopropane, 1-Bromobutane, Bromobenzene
Out of o-and p-dibromobenzene which one has higher melting point and why?
Assertion: The boiling points of alkyl halides decrease in the order:
\[\ce{RI > RBr > RCl > RF}\]
Reason: The boiling points of alkyl chlorides, bromides and iodides are considerably higher than that of the hydrocarbon of comparable molecular mass.
Arrange the following compounds in increasing order of their boiling points:
CH3CH2OH, CH3−CHO, CH3−COOH
Arrange the isomeric dichlorobenzene in the increasing order of their boiling point and melting points.
Write the structure of the following organic halogen compound.
1,4-Dibromobut-2-ene
Write the structure of the following organic halogen compound.
4-tert-Butyl-3-iodoheptane
Write the structure of the following organic halogen compound.
4-tert-Butyl-3-iodoheptane
Write the structure of the following organic halogen compound:
4-tert-Butyl-3-iodoheptane
Write the structure of the following organic halogen compound.
4-tert-Butyl-3-iodoheptane
