Advertisements
Advertisements
प्रश्न
Complete the following nuclear reactions :
(i) `"_15^32P -> ` `"_z^AX + bar(e) + bar(v)`
(ii) `"_6^12 C `+`"_6^12C ->` ` "_2^A Y + ` `"_4^2 He`
उत्तर
`"_15^32P -> ` `"_z^AX + ` ` "_(-1)^0 e + v`
(i) 15 = 2 - 1
∴ Z = 1
A = 0 + 32 = 32
(ii) `"_6^12 C `+`"_6^12C ->` ` "_2^A Y + ` `"_4^2 He`
12 + 12 = A + 4
24 = A + 4
A = 20
6 + 6 =Z + 2
12 = Z + 2
Z = 10
APPEARS IN
संबंधित प्रश्न
Define ‘activity’ of a radioactive material and write its S.I. units.
Plot a graph showing variation of activity of a given radioactive sample with time.
The sequence of stepwise decay of a radioactive nucleus is
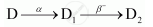
If the atomic number and mass number of D2 are 71 and 176 respectively, what are their corresponding values of D?
The half-life of 199Au is 2.7 days. (a) Find the activity of a sample containing 1.00 µg of 198Au. (b) What will be the activity after 7 days? Take the atomic weight of 198Au to be 198 g mol−1.
The count rate from a radioactive sample falls from 4.0 × 106 per second to 1.0 × 106per second in 20 hours. What will be the count rate 100 hours after the beginning?
The half-life of 226Ra is 1602 y. Calculate the activity of 0.1 g of RaCl2 in which all the radium is in the form of 226Ra. Taken atomic weight of Ra to be 226 g mol−1 and that of Cl to be 35.5 g mol−1.
A vessel of volume 125 cm3 contains tritium (3H, t1/2 = 12.3 y) at 500 kPa and 300 K. Calculate the activity of the gas.
Natural water contains a small amount of tritium (`""_1^3H`). This isotope beta-decays with a half-life of 12.5 years. A mountaineer while climbing towards a difficult peak finds debris of some earlier unsuccessful attempt. Among other things he finds a sealed bottled of whisky. On returning, he analyses the whisky and finds that it contains only 1.5 per cent of the `""_1^3H` radioactivity as compared to a recently purchased bottle marked '8 years old'. Estimate the time of that unsuccessful attempt.
A human body excretes (removes by waste discharge, sweating, etc.) certain materials by a law similar to radioactivity. If technetium is injected in some form in a human body, the body excretes half the amount in 24 hours. A patient is given an injection containing 99Tc. This isotope is radioactive with a half-life of 6 hours. The activity from the body just after the injection is 6 μCi. How much time will elapse before the activity falls to 3 μCi?
The half-life of a certain radioactive element is 3.465 days. Find its disintegration constant.
