Advertisements
Advertisements
प्रश्न
Draw the electron dot structures for propanone.
उत्तर
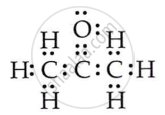
OR
\[\begin{array}{cc}
\phantom{}\ce{H}\phantom{...}\ce{O}\phantom{...}\ce{H}\phantom{}\\
\phantom{}|\phantom{....}||\phantom{....}|\phantom{}\\
\ce{H - C - C - C - H}\\
\phantom{}|\phantom{.........}|\phantom{}\\
\phantom{}\ce{H}\phantom{........}\ce{H}\phantom{}
\end{array}\]
APPEARS IN
संबंधित प्रश्न
Give a chemical test to distinguish between saturated and unsaturated hydrocarbons.
Why does carbon form strong bonds with most other elements ?
What will be the formula and electron dot structure of cyclopentane?
Write the electron-dot structures for ethene.
Write the electron-dot structures for ethyne.
Out of the following pairs of compounds, the unsaturated compounds are:
(a) C2H6 and C4H6
(b) C6H12 and C5H12
(c) C4H6 and C6H12
(d) C2H6 and C4H10
Name two catalysts which can be used in the hydrogenation of unsaturated compounds.
Which of the following will give addition reaction and why?
C4H10, C2H6, C2H4, CH4, C3H8, C3H4
Name a chemical reaction which is characteristic of unsaturated hydrocarbons (like alkenes and alkynes).
Structural formula of ethyne is ______.
In which of the following compounds, — OH is the functional group?
Structural formula of ethyne is
Which among the following are unsaturated hydrocarbons?
- \[\ce{H3C - CH2 - CH2 - CH3}\]
- \[\ce{H3C - C ≡ C - CH3}\]
- \[\begin{array}{cc}
\ce{H3C - CH - CH3}\\
|\phantom{..}\\
\ce{CH3}\\
\end{array}\] - \[\begin{array}{cc}
\ce{H3C - C = CH2}\\
|\\
\phantom{...}\ce{CH3}\\
\end{array}\]
Identify and name the functional groups present in the following compounds.
- \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{..}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{..}\\
\ce{H - C - C - C - OH}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{..}\\
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{..}\\
\end{array}\] - \[\begin{array}{cc}
\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{....}\\
\phantom{...}|\phantom{....}|\phantom{....}||\phantom{....}\\
\ce{H - C - C - C - OH}\\
\phantom{...}|\phantom{....}|\phantom{.........}\\
\phantom{}\ce{H}\phantom{...}\ce{H}\phantom{.......}\\
\end{array}\] - \[\begin{array}{cc}
\phantom{......}\ce{H}\phantom{....}\ce{H}\phantom{....}\ce{O}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{.......}\\
\phantom{....}|\phantom{....}|\phantom{....}||\phantom{....}|\phantom{....}|\phantom{....}\\
\ce{H} - \ce{C} - \ce{C} - \ce{C} - \ce{C} - \ce{C} - \ce{H}\\
\phantom{....}|\phantom{.....}|\phantom{........}|\phantom{....}|\phantom{....}\\
\phantom{....}\ce{H}\phantom{....}\ce{H}\phantom{........}\ce{H}\phantom{..}\ce{H}\phantom{.....}\\
\end{array}\] - \[\begin{array}{cc}
\phantom{......}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{......}\\
\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}\\
\ce{H - C - C - C = C - H}\\
\phantom{....}|\phantom{....}|\phantom{.............}\\
\phantom{....}\ce{H}\phantom{...}\ce{H}\phantom{.............}
\end{array}\]
What is the role of metal or reagents written on arrows in the given chemical reactions?
- \[\begin{array}{cc}
\ce{CH3}\phantom{.....}\ce{CH3}\phantom{.........}\ce{CH3}\phantom{..}\ce{CH3}\phantom{..........}\\
\phantom{...}\backslash\phantom{......}/\phantom{................}|\phantom{.....}|\phantom{............}\\
\ce{C} = \ce{C} + \ce{H2} \overset{\ce{Ni}}{\rightarrow} \ce{CH3 - C - C - CH3}\\
\phantom{.}/\phantom{........}\backslash\phantom{..............}|\phantom{....}|\phantom{.........}\\
\ce{CH3}\phantom{....}\ce{CH3}\phantom{............}\ce{H}\phantom{...}\ce{H}\phantom{........}
\end{array}\] - \[\ce{CH3COOH + CH3CH2OH \overset{\ce{Conc. H2SO4}}{\rightarrow}CH3COOC2H5 + H2O}\]
- \[\ce{CH3CH2OH}\ce{->[Alk.KMnO4][Heat]CH3COOH}\]
Name the reaction which is commonly used in the conversion of vegetable oils to fats. Explain the reaction involved in detail.
Write the name of the following compound
\[\begin{array}{cc}
\phantom{}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{.....}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{.....}\\
\ce{H} - \ce{C} - \ce{C} - \ce{C} ≡ \ce{C} - \ce{H}\\
\phantom{}|\phantom{....}|\phantom{..........}\\
\phantom{}\ce{H}\phantom{...}\ce{H}\phantom{..........}\\
\end{array}\]
Write the name of the following compound
\[\begin{array}{cc}
\phantom{}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{}\\
\ce{H - C - C - C - C - C - C - C = O}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{.....}\\
\phantom{.}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{......}
\end{array}\]
Draw the electron dot structure for ethyne.
