Advertisements
Advertisements
Question
Draw the electron dot structures for propanone.
Solution
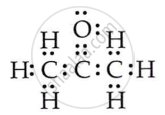
OR
\[\begin{array}{cc}
\phantom{}\ce{H}\phantom{...}\ce{O}\phantom{...}\ce{H}\phantom{}\\
\phantom{}|\phantom{....}||\phantom{....}|\phantom{}\\
\ce{H - C - C - C - H}\\
\phantom{}|\phantom{.........}|\phantom{}\\
\phantom{}\ce{H}\phantom{........}\ce{H}\phantom{}
\end{array}\]
APPEARS IN
RELATED QUESTIONS
Write the number of covalent bonds in the molecule of ethane.
Explain in brief two main reasons for carbon forming a large number of compounds.
What will be the formula and electron dot structure of cyclopentane?
Draw the electron dot structures for F2.
Give a test that can be used to differentiate chemically between butter and cooking oil.
Write a short note on Catenation.
Fill in the following blank with suitable word:
Ethyne has ......... carbon-hydrogen single bonds.
Giving their structures, state the number of single bonds, double bonds and triple bonds (if any) in the following compounds:
ethene
Give the names and structural formulae of one saturated cyclic hydrocarbon and one unsaturated cyclic hydrocarbon.
A cyclic hydrocarbon having carbon-carbon single bonds as well as carbon-carbon double bonds in its molecule is:
(a) C6H12
(b) C6H14
(c) C6H6
(d) C6H10
You are given the following molecular formulae of some hydrocarbons:
C5H8; C7H14; C6H6; C5H10; C7H12; C6H12
Which two formulae represent unsaturated hydrocarbons having triple bonds?
Which of the following will give addition reaction and why?
C4H10, C2H6, C2H4, CH4, C3H8, C3H4
A mixture of ethyne (acetylene) and oxygen is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
Which of the two is better for our health : butter or vegetable oil? Why?
Name the reaction which is usually used in the conversion of vegetables oils to fats. Explain the reaction involved in detail. Write a chemical equation to illustrate your answer.
The correct electron dot structure of a water molecule is
Write the name of the following compound
\[\begin{array}{cc}
\phantom{....}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{O}\phantom{......}\\
\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}||\phantom{......}\\
\ce{H} - \ce{C} - \ce{C} - \ce{C} - \ce{C} - \ce{C} - \ce{OH}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{.......}\\
\phantom{}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{.......}\\
\end{array}\]
What is the role of metal or reagents written on arrows in the given chemical reactions?
- \[\begin{array}{cc}
\ce{CH3}\phantom{.....}\ce{CH3}\phantom{.........}\ce{CH3}\phantom{..}\ce{CH3}\phantom{..........}\\
\phantom{...}\backslash\phantom{......}/\phantom{................}|\phantom{.....}|\phantom{............}\\
\ce{C} = \ce{C} + \ce{H2} \overset{\ce{Ni}}{\rightarrow} \ce{CH3 - C - C - CH3}\\
\phantom{.}/\phantom{........}\backslash\phantom{..............}|\phantom{....}|\phantom{.........}\\
\ce{CH3}\phantom{....}\ce{CH3}\phantom{............}\ce{H}\phantom{...}\ce{H}\phantom{........}
\end{array}\] - \[\ce{CH3COOH + CH3CH2OH \overset{\ce{Conc. H2SO4}}{\rightarrow}CH3COOC2H5 + H2O}\]
- \[\ce{CH3CH2OH}\ce{->[Alk.KMnO4][Heat]CH3COOH}\]
Write the name of the following compound
\[\begin{array}{cc}
\phantom{}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{}\\
\ce{H - C - C - C - C - C - C - C = O}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{.....}\\
\phantom{.}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{......}
\end{array}\]
Write the name of the following compound
\[\begin{array}{cc}
\phantom{}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{..}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{..}\\
\ce{H - C - C - C - C - C - OH}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{..}\\
\phantom{}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{..}
\end{array}\]
