Advertisements
Advertisements
Question
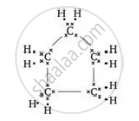
What will be the formula and electron dot structure of cyclopentane?
Solution
The formula for cyclopentane is \[\ce{C5H10}\]. Its electron dot structure is given below.

APPEARS IN
RELATED QUESTIONS
Explain in brief two main reasons for carbon forming a large number of compounds.
How many structural isomers can you draw for pentane?
Draw the electron dot structures for H2S.
Draw the electron dot structures for propanone.
Give a test that can be used to differentiate chemically between butter and cooking oil.
A compound 'X' on heating with excess conc. sulphuric acid at 443 K gives an unsaturated compound 'Y'. 'X' also reacts with sodium metal to evolve a colourless gas 'Z'. Identify 'X', 'Y' and 'Z'. Write the equation of the chemical reaction of formation of 'Y' and also write the role of sulphuric acid in the reaction.
Write the electron-dot structures for ethene.
Giving their structures, state the number of single bonds, double bonds and triple bonds (if any) in the following compounds:
ethyne
Out of the following pairs of compounds, the unsaturated compounds are:
(a) C2H6 and C4H6
(b) C6H12 and C5H12
(c) C4H6 and C6H12
(d) C2H6 and C4H10
Name a chemical reaction which is characteristic of unsaturated hydrocarbons (like alkenes and alkynes).
Name the reaction which is usually used in the conversion of vegetables oils to fats. Explain the reaction involved in detail. Write a chemical equation to illustrate your answer.
Differentiate between alkanes and alkenes. Name and draw the structure of one member of each.
Explain the following reaction with balanced chemical equation:
Calcium reacts with water.
Which of the following statements are usually correct for carbon compounds? These
- are good conductors of electricity
- are poor conductors of electricity
- have strong forces of attraction between their molecules
- do not have strong forces of attraction between their molecules
Structural formula of ethyne is
Which among the following are unsaturated hydrocarbons?
- \[\ce{H3C - CH2 - CH2 - CH3}\]
- \[\ce{H3C - C ≡ C - CH3}\]
- \[\begin{array}{cc}
\ce{H3C - CH - CH3}\\
|\phantom{..}\\
\ce{CH3}\\
\end{array}\] - \[\begin{array}{cc}
\ce{H3C - C = CH2}\\
|\\
\phantom{...}\ce{CH3}\\
\end{array}\]
Name the reaction which is commonly used in the conversion of vegetable oils to fats. Explain the reaction involved in detail.
Write the name of the following compound
\[\begin{array}{cc}
\phantom{}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{.....}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{.....}\\
\ce{H} - \ce{C} - \ce{C} - \ce{C} ≡ \ce{C} - \ce{H}\\
\phantom{}|\phantom{....}|\phantom{..........}\\
\phantom{}\ce{H}\phantom{...}\ce{H}\phantom{..........}\\
\end{array}\]
Draw the electron dot structure for ethyne.
