Advertisements
Advertisements
प्रश्न
Draw the electron dot structures for propanone.
उत्तर
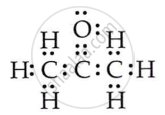
OR
\[\begin{array}{cc}
\phantom{}\ce{H}\phantom{...}\ce{O}\phantom{...}\ce{H}\phantom{}\\
\phantom{}|\phantom{....}||\phantom{....}|\phantom{}\\
\ce{H - C - C - C - H}\\
\phantom{}|\phantom{.........}|\phantom{}\\
\phantom{}\ce{H}\phantom{........}\ce{H}\phantom{}
\end{array}\]
APPEARS IN
संबंधित प्रश्न
‘A’ is an element having four electrons in its outermost orbit. An allotropes ‘B’ of this element is used as a dry lubricant in machinery and in pencil leads. So:
i. Write the name of element ‘A’ and its allotropes.
ii. State whether ‘B’ is a good conductor or non-conductor of electricity?
What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
Write a short note on Catenation.
Write the electron-dot structures for ethyne.
Fill in the following blank with suitable word:
Ethyne has ......... carbon-hydrogen single bonds.
Give the names and structural formulae of one saturated cyclic hydrocarbon and one unsaturated cyclic hydrocarbon.
Out of the following pairs of compounds, the unsaturated compounds are:
(a) C2H6 and C4H6
(b) C6H12 and C5H12
(c) C4H6 and C6H12
(d) C2H6 and C4H10
An unsaturated hydrocarbon having a triple covalent bond has 50 hydrogen atoms in its molecule. The number of carbon atoms in its molecule will be:
(a) 24
(b) 25
(c) 26
(d) 28
Write the molecular formula of an alkene having 20 carbon atoms?
Name the reaction which is usually used in the conversion of vegetables oils to fats. Explain the reaction involved in detail. Write a chemical equation to illustrate your answer.
Fill in the blanks and rewrite the completed statements:
The organic compounds having double or triple bond in them are termed as _________________ _________________.
Which among the following are unsaturated hydrocarbons?
- \[\ce{H3C - CH2 - CH2 - CH3}\]
- \[\ce{H3C - C ≡ C - CH3}\]
- \[\begin{array}{cc}
\ce{H3C - CH - CH3}\\
|\phantom{..}\\
\phantom{.}\ce{CH3}
\end{array}\] - \[\begin{array}{cc}
\ce{H3C - C = CH2}\\
|\phantom{.}\\
\phantom{..}\ce{CH3}
\end{array}\]
Identify the unsaturated compounds from the following:
- Propane
- Propene
- Propyne
- Chloropropane
Which of the following is the correct representation of electron dot structure of nitrogen?
What is the role of metal or reagents written on arrows in the given chemical reactions?
- \[\begin{array}{cc}
\ce{CH3}\phantom{.....}\ce{CH3}\phantom{.........}\ce{CH3}\phantom{..}\ce{CH3}\phantom{..........}\\
\phantom{...}\backslash\phantom{......}/\phantom{................}|\phantom{.....}|\phantom{............}\\
\ce{C} = \ce{C} + \ce{H2} \overset{\ce{Ni}}{\rightarrow} \ce{CH3 - C - C - CH3}\\
\phantom{.}/\phantom{........}\backslash\phantom{..............}|\phantom{....}|\phantom{.........}\\
\ce{CH3}\phantom{....}\ce{CH3}\phantom{............}\ce{H}\phantom{...}\ce{H}\phantom{........}
\end{array}\] - \[\ce{CH3COOH + CH3CH2OH \overset{\ce{Conc. H2SO4}}{\rightarrow}CH3COOC2H5 + H2O}\]
- \[\ce{CH3CH2OH}\ce{->[Alk.KMnO4][Heat]CH3COOH}\]
- What are hydrocarbons? Give examples.
- Give the structural differences between saturated and unsaturated hydrocarbons with two examples each.
- What is a functional group? Give examples of four different functional groups.
Write the name of the following compound
\[\begin{array}{cc}
\phantom{}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{.....}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{.....}\\
\ce{H} - \ce{C} - \ce{C} - \ce{C} ≡ \ce{C} - \ce{H}\\
\phantom{}|\phantom{....}|\phantom{..........}\\
\phantom{}\ce{H}\phantom{...}\ce{H}\phantom{..........}\\
\end{array}\]
Write the name of the following compound
\[\begin{array}{cc}
\phantom{}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{}\\
\ce{H - C - C - C - C - C - C - C = O}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{.....}\\
\phantom{.}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{......}
\end{array}\]
