Advertisements
Advertisements
प्रश्न
Explain resonance with reference to a carbonate ion.
उत्तर
It is evident from the experimental results that all carbon-oxygen bonds in carbonate ions are equivalent. The actual structure of the molecules is said to be resonance hybrid, an average of these three resonance forms. It is important to note that carbonate ion does not change from one structure to another and vice versa. is not possible to picturise the resonance hybrid by drawing a single Lewis structure. However, the following structure gives a qualitative idea about the correct structure.

Resonance structure of CO32-
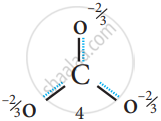
Resonance Hybrid structure of CO32-
It is found that the energy of the resonance hybrid (structure 4) is lower than that of all possible canonical structures (Structure 1, 2 & 3). The difference in energy between structure 1 or 2 or 3, (most stable canonical structure) and structure 4 (resonance hybrid) is called resonance energy.
APPEARS IN
संबंधित प्रश्न
Bond order of a species is 2.5 and the number of electons in its bonding molecular orbital is formd to be 8 The no. of electons in its antibonding molecular orbital is
What is dipole moment?
Linear form of carbondioxide molecule has two polar bonds. Yet the molecule has Zero dipole moment. Why?
Define bond energy.
Explain the bond formation in ethylene.
Explain the bond formation in acetylene.
CO2 and H2O both are triatomic molecule but their dipole moment values are different. Why?
Which one of the following has the highest bond order?
- N2
- N2+
- N2–
Describe Fajan’s rule.
The correct sequence of decrease in the bond angles of the following hydrides is.
