Advertisements
Advertisements
प्रश्न
Explain the following observation.
The type of an automobile is inflated to slightly lesser pressure in summer than in winter
उत्तर
The pressure of air is directly proportional to the temperature. Since the temperature is higher in summer than in higher, the pressure of the air in the tube of the lyre is likely to be quite high as compared to winter. It is quite likely that the tube may burst under high pressure in summer, Therefore, it is advisable to inflate the types to lesser pressure in summer than in winter.
APPEARS IN
संबंधित प्रश्न
Convert the following temperature from degree Celcius to kelvin.
273° C
Convert exactly 1.5 atm to pascals
Convert 0.124 torr to the standard atmosphere
Identify the gas laws from the following diagram.
| Diagram | Gas laws |
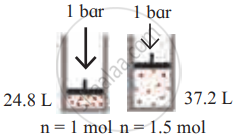 |
______________ |
With the help of the graph answer the following -

At constant temperature, the Graph shows the relationship between pressure and volume. Represent the relation mathematically.
Use of hot air balloon in sports and meteorological observation is an application of
Explain the following observation.
The size of a weather balloon becomes larger and larger as it ascends up to larger altitude
According to Andrews isothermals, the minimum temperature at which carbon dioxide gas obeys Boyles law is ______.
Volume of a balloon at 25°C and 1 bar pressure is 2.27 L. If the pressure of the gas in balloon is reduced to 0.227 bar, what is the rise in volume of a gas?
A gas occupies a volume of 4.2 dm3 at 101 kPa pressure. What volume will gas occupy if the pressure is increased to 235 kPa keeping the temperature constant?
