Advertisements
Advertisements
प्रश्न
Identify the gas laws from the following diagram.
| Diagram | Gas laws |
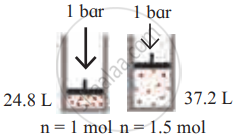 |
______________ |
उत्तर
| Diagram | Gas laws |
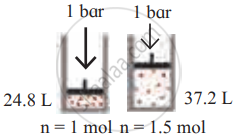 |
Avogadro’s law |
APPEARS IN
संबंधित प्रश्न
State (i) the three variables for gas laws and (ii) SI units of these variables.
Answer in one sentence.
A bubble of methane gas rises from the bottom of the North sea. What will happen to the size of the bubble as it rises to the surface?
Convert the following temperature from degree Celcius to kelvin.
273° C
Convert the following pressure value into Pascals.
10 atmosphere
Convert 0.124 torr to the standard atmosphere
Identify the gas laws from the following diagram.
| Diagram | Gas laws |
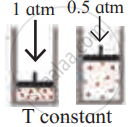 |
______________ |
Identify the gas laws from the following diagram.
| Diagram | Gas laws |
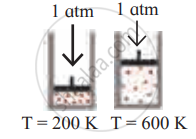 |
______________ |
Consider a sample of a gas in a cylinder with a movable piston.
Show diagrammatically the changes in the position of the piston, if pressure is increased from 1.0 bar to 2.0 bar at a constant temperature.
Consider a sample of a gas in a cylinder with a movable piston.

Show diagrammatically the changes in the position of the piston, if the temperature is decreased from 400 K to 300 K, and pressure is decreased from 4 bar to 3 bar.
Match the pairs of the following:
| Column ‘A’ | Column ‘B’ |
| a. Boyle’s law | i. at constant pressure and volume |
| b. Charles’ law | ii. at constant temperature |
| iii. at constant pressure |
With the help of the graph answer the following -

At constant temperature, the Graph shows the relationship between pressure and volume. Represent the relation mathematically.
With the help of the graph answer the following -

At constant temperature, Write the statement of law.
Solve the following.
A syringe has a volume of 10.0 cm3 at pressure 1 atm. If you plug the end so that no gas can escape and push the plunger down, what must be the final volume to change the pressure to 3.5 atm?

Solve the following.
The volume of a given mass of a gas at 0°C is 2 dm3. Calculate the new volume of the gas at constant pressure when the temperature is increased by 10°C.
Solve the following.
The volume of a given mass of a gas at 0°C is 2 dm3. Calculate the new volume of the gas at constant pressure when the temperature is decreased by 10°C.
Use of hot air balloon in sports and meteorological observation is an application of
Assertion: Critical temperature of CO2 is 304 K, it can be liquefied above 304 K.
Reason: For a given mass of gas, volume is to directly proportional to pressure at constant temperature
State Boyle's law.
Give the mathematical expression that relates gas volume and moles.
Explain the following observation.
Aerated water bottles are kept under water during summer
A sample of gas at 15°C at 1 atm. has a volume of 2.58 dm3. When the temperature is raised to 38°C at 1 atm does the volume of the gas Increase? If so, calculate the final volume.
Sulphur hexafluoride is a colourless, odourless gas; calculate the pressure exerted by 1.82 moles of the gas in a steel vessel of volume 5.43 dm3 at 69.5 °C, assuming ideal gas behaviour
At 25°C and 1 atm, a cylinder containing 10 L of an ideal gas is connected to the empty cylinder with a capacity of 20 L. The pressures exerted by gas m both the cylinders will be ____________.
A gas occupies a volume of 4.2 dm3 at 101 kPa pressure. What volume will gas occupy if the pressure is increased to 235 kPa keeping the temperature constant?
10 g of gas at one atomospheric pressure is cooled from 273.15°C to 0°C keeping the volume constant. What is the final pressure?
If 300 mL of a gas at 26.85°C is cooled to 6.85°C at constant pressure. What will be the final volume of gas?
At what temperature, the volume of gas would become zero?
