Advertisements
Advertisements
प्रश्न
Sulphur hexafluoride is a colourless, odourless gas; calculate the pressure exerted by 1.82 moles of the gas in a steel vessel of volume 5.43 dm3 at 69.5 °C, assuming ideal gas behaviour
उत्तर
n = 1.82 mole
V = 5.43 dm3
T = 69.5 + 273 = 342.5
P = ?
PV = nRT
P = `"nRT"/"V"`
P = `(1.82 "mol" xx 0.0821 "dm"^3 "atm mol"^-1 "K"^-1 xx 342.5 "K")/(5.43 "dm"^3)`
P = 9.425 atm
APPEARS IN
संबंधित प्रश्न
Convert the following temperature from degree Celcius to kelvin.
−15° C
Convert the following temperature from degree Celcius to kelvin.
−197° C
Convert the following pressure value into Pascals.
10 atmosphere
Convert 101.325 kPa to bar.
Consider a sample of a gas in a cylinder with a movable piston.
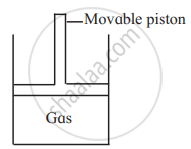
Show diagrammatically the changes in the position of the piston, if pressure is increased from 1.0 bar to 2.0 bar at a constant temperature.
Write the statement for Charles’ law
Solve the following.
A balloon is inflated with helium gas at room temperature of 25°C and at 1 bar pressure when its initial volume is 2.27L and allowed to rise in the air. As it rises in the air external pressure decreases and the volume of the gas increases till finally, it bursts when external pressure is 0.3bar. What is the limit at which the volume of the balloon can stay inflated?
According to Andrews isothermals, the minimum temperature at which carbon dioxide gas obeys Boyles law is ______.
If 2 moles of an ideal gas at 546 K has volume of 44.8 L, then what will be it's pressure? (R = 0.082)
The number of molecules in 8.96 litres of gas at 0°C and 1 atm. pressure is approximately ______.
