Advertisements
Advertisements
प्रश्न
Of two samples of nitrogen gas, sample A contains 1.5 moles of nitrogen in a vessel of the volume of 37.6 dm3 at 298 K, and sample B is in a vessel of volume 16.5 dm3 at 298 K. Calculate the number of moles in sample B.
उत्तर
nA = 1.5 mol
nB = ?
VA = 37.6 dm3
VB = 16.5 dm3
(T = 298 K constant)
`"V"_"A"/"n"_"A" = "V"_"B"/"n"_"B"`
`37.6/1.5 = 16.5/"n"_"B"`
`25.06 = 16.5/"n"_"B"`
`"n"_"B" = 16.5/25.06`
`"n"_"B"` = 0.658 mol
∴ The number of moles in sample B is 0.658 mol
APPEARS IN
संबंधित प्रश्न
Explain Why?
"When stating the volume of a gas, the pressure and temperature should also be given."
Convert the following temperature from degree Celcius to kelvin.
−15° C
Identify the gas laws from the following diagram.
| Diagram | Gas laws |
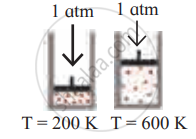 |
______________ |
Write the statement for Boyle’s law
Write the statement for Charles’ law
Solve the following.
At 0°C, a gas occupies 22.4 liters. How much hot must be the gas in celsius and in kelvin to reach a volume of 25.0 liters?
For a given mass of an ideal gas, which of the following statements is CORRECT?
Isochor is the graph plotted between ______.
If 2 moles of an ideal gas at 546 K has volume of 44.8 L, then what will be it's pressure? (R = 0.082)
If 300 mL of a gas at 26.85°C is cooled to 6.85°C at constant pressure. What will be the final volume of gas?
