Advertisements
Advertisements
प्रश्न
Explain the following:
Polar covalent compounds conduct electricity?
उत्तर
Polar covalent compounds conduct electricity because they form ions in their solutions and hence dissociate in water.
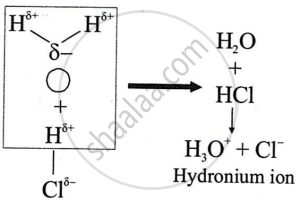
APPEARS IN
संबंधित प्रश्न
What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur? (Hint – the eight atoms of sulphur are joined together in the form of a ring.)
How will you find out which of the water soluble compound A or B is ionic?
The solution of one of the following compounds will not conduct electricity. This compounds is:
(a) NaCl
(b) CCl4
(c) MgCl2
(d) CaCl2
Explain the following:
Non-polar covalent compounds are insoluble in water.
The following table shows the electronic configuration of the elements W, X, Y, Z:
|
Element |
W |
X |
Y |
Z |
|
Electronic |
2,8,1 |
2,8,7 |
2,5 |
1 |
Answer the following questions based on the table above:
What type of bond is formed between Y and Z.
Elements Q and S react together to form an ionic compound. Under normal conditions, which physical state will the compound QS exist in?
What is the difference between :
Ionic compounds and covalent compounds
Potassium (Atomic No. 19) and chlorine (Atomic No. 17) react to form a compound. On the basis of electronic concept, explain
(i) oxidation
(ii) reduction
(iii)oxidising agent
(iv)reducing agent
Number of valence electrons in a carbon atom is _______.
When ethyl alcohol and acetic acid are mixed, the resulting ester has a chemical formula ______.
