Advertisements
Advertisements
प्रश्न
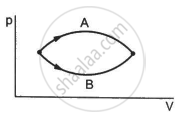
Figure shows the P-V diagram of an ideal gas undergoing a change of state from A to B. Four different parts I, II, III and IV as shown in the figure may lead to the same change of state.

- Change in internal energy is same in IV and III cases, but not in I and II.
- Change in internal energy is same in all the four cases.
- Work done is maximum in case I
- Work done is minimum in case II.
उत्तर
b and c
Explanation:
Change in internal energy depends only upon the initial and final positions and not on the path followed. Hence, the change in internal energy is the same for all the cases. The work done is dependent upon the path followed. Work done is the area enclosed by the path with the x-axis. Since path I enclose the maximum area with the x-axis, the work done is maximum in case I.
APPEARS IN
संबंधित प्रश्न
In changing the state of a gas adiabatically from an equilibrium state A to another equilibrium state B, an amount of work equal to 22.3 J is done on the system. If the gas is taken from state A to B via a process in which the net heat absorbed by the system is 9.35 cal, how much is the net work done by the system in the latter case? (Take 1 cal = 4.19 J)
Figure shows two processes A and B on a system. Let ∆Q1 and ∆Q2 be the heat given to the system in processes A and B respectively. Then ____________ .
A gas is initially at a pressure of 100 kPa and its volume is 2.0 m3. Its pressure is kept constant and the volume is changed from 2.0 m3 to 2.5 m3. Its Volume is now kept constant and the pressure is increased from 100 kPa to 200 kPa. The gas is brought back to its initial state, the pressure varying linearly with its volume. (a) Whether the heat is supplied to or extracted from the gas in the complete cycle? (b) How much heat was supplied or extracted?
derive the relation between the change in internal energy (∆U), work is done (W), and heat (Q).
The internal energy of a system is ______
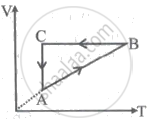
A cyclic process ABCA is shown in the V-T diagram. A process on the P-V diagram is ______.
If a gas is compressed adiabatically:
The internal energy of one mole of argon is ______.
A steam engine delivers 4.8 x 108 Jof work per minute and services 1.2 x 109 J of heat per minute from its boiler. What is the percentage efficiency of the engine?
Explain the change in internal energy of a thermodynamic system (the gas) by heating it.
