Advertisements
Advertisements
प्रश्न
Explain the change in internal energy of a thermodynamic system (the gas) by heating it.
उत्तर
Consider a gas filled in a cylinder fitted with a massless, movable, and frictionless piston as shown in the figure.
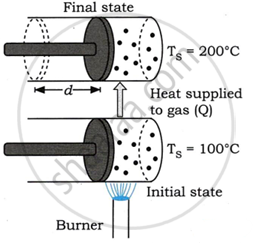
Let Ts be the temperature of gas (system),
TE be temperature of environment.
Initially, the cylinder is heated using a burner, as illustrated in the picture. At this level, TE exceeds Ts. The temperature difference between the source of heat and the system causes energy to flow towards the gas in the cylinder. This increases the gas's internal energy. When the environment is cooler than the gas, Ts > TE, resulting in energy transfer from the gas to the surroundings.
The gas expands as a result of the piston being forced out during this procedure. The gas does a certain amount of work. The gas cools, and some of its energy is lost. This describes how exerting effort can alter a gas's intrinsic energy.
APPEARS IN
संबंधित प्रश्न
Explain why Two bodies at different temperatures T1 and T2, if brought in thermal contact, do not necessarily settle to the mean temperature (T1 + T2)/2.
Two cylinders A and B of equal capacity are connected to each other via a stopcock. A contains a gas at standard temperature and pressure. B is completely evacuated. The entire system is thermally insulated. The stopcock is suddenly opened. Answer the following:
Do the intermediate states of the system (before settling to the final equilibrium state) lie on its P-V-T surface?
A force F is applied on a block of mass M. The block is displaced through a distance d in the direction of the force. What is the work done by the force on the block? Does the internal energy change because of this work?
The outer surface of a cylinder containing a gas is rubbed vigorously by a polishing machine. The cylinder and its gas become warm. Is the energy transferred to the gas heat or work?
Can work be done by a system without changing its volume?
Consider the following two statements.
(A) If heat is added to a system, its temperature must increase.
(B) If positive work is done by a system in a thermodynamic process, its volume must increase.
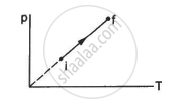
An ideal gas goes from the state i to the state f as shown in figure. The work done by the gas during the process ______________ .
A gas is contained in a metallic cylinder fitted with a piston. The piston is suddenly moved in to compress the gas and is maintained at this position. As time passes the pressure of the gas in the cylinder ______________ .
The pressure p and volume V of an ideal gas both increase in a process.
(a) Such a process is not possible.
(b) The work done by the system is positive.
(c) The temperature of the system must increase.
(d) Heat supplied to the gas is equal to the change in internal energy.
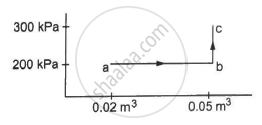
A substance is taken through the process abc as shown in figure. If the internal energy of the substance increases by 5000 J and a heat of 2625 cal is given to the system, calculate the value of J.
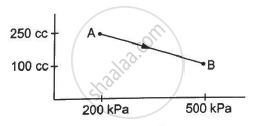
A gas is taken along the path AB as shown in figure. If 70 cal of heat is extracted from the gas in the process, calculate the change in the internal energy of the system.
A mixture of fuel and oxygen is burned in a constant-volume chamber surrounded by a water bath. It was noticed that the temperature of water is increased during the process. Treating the mixture of fuel and oxygen as the system,
- Has heat been transferred?
- Has work been done?
- What is the sign of ∆U?
A system releases 130 kJ of heat while 109 kJ of work is done on the system. Calculate the change in internal energy.
Which of the following is correct, when the energy is transferred to a system from its environment?
What is the energy associated with the random, disordered motion of the molecules of a system called as?
When does a system lose energy to its surroundings and its internal energy decreases?
A system releases 100 kJ of heat while 80 kJ of work is done on the system. Calculate the change in internal energy.
Explain given cases related to energy transfer between the system and surrounding –
- energy transferred (Q) > 0
- energy transferred (Q) < 0
- energy transferred (Q) = 0
Explain the different ways through which the internal energy of the system can be changed.
A cylinder containing one gram molecule of the gas was compressed adiabatically until its temperature rose from 27°C to 97°C. Calculate the work done and heat produced in the gas (𝛾 = 1.5).
In insulated systems, the amount of external work done by the gas is proportional to:
Figure shows the P-V diagram of an ideal gas undergoing a change of state from A to B. Four different parts I, II, III and IV as shown in the figure may lead to the same change of state.

- Change in internal energy is same in IV and III cases, but not in I and II.
- Change in internal energy is same in all the four cases.
- Work done is maximum in case I
- Work done is minimum in case II.
n mole of a perfect gas undergoes a cyclic process ABCA (see figure) consisting of the following processes:
A `→` B: Isothermal expansion at temperature T so that the volume is doubled from V1 to V2 = 2V1 and pressure changes from P1 to P2.
B `→` C: Isobaric compression at pressure P2 to initial volume V1.
C `→` A: Isochoric change leading to change of pressure from P2 to P1.
Total workdone in the complete cycle ABCA is ______.

The internal energy of one mole of argon is ______.
A steam engine delivers 4.8 x 108 Jof work per minute and services 1.2 x 109 J of heat per minute from its boiler. What is the percentage efficiency of the engine?
A system releases 125 kJ of heat while 104 kJ work is done on the system. Calculate the change in internal energy.
