Advertisements
Advertisements
प्रश्न
Explain the different ways through which the internal energy of the system can be changed.
उत्तर
- The internal energy of a system can be changed by changing the temperature of the system:
a. If the system is placed in an environment that is at a temperature lower than the system, i.e., TS > TE, the energy is transferred from the system to the environment causing a decrease in the internal energy of the system.
b. If the system is placed in an environment that is at a temperature higher than the system, i.e., TE > TS, the energy is transferred from the environment to the system causing the increase in the internal energy of the system. - The internal energy of a system can be changed by doing some work:
a. When some work is done on the system by the environment, the system gains energy, and its temperature increases causing an increase in internal energy.
b. When some work is done by the system on the environment, the system loses the energy, and its temperature decreases causing a decrease in internal energy.
APPEARS IN
संबंधित प्रश्न
Explain why Air pressure in a car tyre increases during driving.
The outer surface of a cylinder containing a gas is rubbed vigorously by a polishing machine. The cylinder and its gas become warm. Is the energy transferred to the gas heat or work?
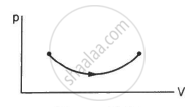
Consider the process on a system shown in figure. During the process, the work done by the system ______________ .
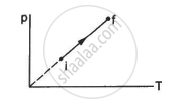
An ideal gas goes from the state i to the state f as shown in figure. The work done by the gas during the process ______________ .
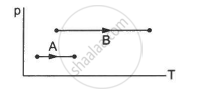
Consider two processes on a system as shown in figure.
The volumes in the initial states are the same in the two processes and the volumes in the final states are also the same. Let ∆W1 and ∆W2 be the work done by the system in the processes A and B respectively.
A gas is contained in a metallic cylinder fitted with a piston. The piston is suddenly moved in to compress the gas and is maintained at this position. As time passes the pressure of the gas in the cylinder ______________ .
A 100 kg lock is started with a speed of 2.0 m s−1 on a long, rough belt kept fixed in a horizontal position. The coefficient of kinetic friction between the block and the belt is 0.20. (a) Calculate the change in the internal energy of the block-belt system as the block comes to a stop on the belt. (b) Consider the situation from a frame of reference moving at 2.0 m s−1 along the initial velocity of the block. As seen from this frame, the block is gently put on a moving belt and in due time the block starts moving with the belt at 2.0 m s−1. calculate the increase in the kinetic energy of the block as it stops slipping past the belt. (c) Find the work done in this frame by the external force holding the belt.
A gas is taken through a cyclic process ABCA as shown in figure. If 2.4 cal of heat is given in the process, what is the value of J ?
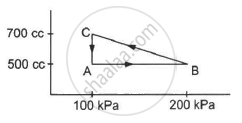
A substance is taken through the process abc as shown in figure. If the internal energy of the substance increases by 5000 J and a heat of 2625 cal is given to the system, calculate the value of J.
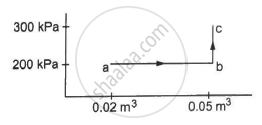
A gas is taken along the path AB as shown in figure. If 70 cal of heat is extracted from the gas in the process, calculate the change in the internal energy of the system.
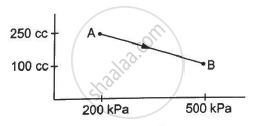
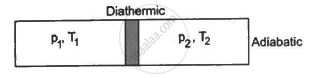
Figure shows a cylindrical tube of volume V with adiabatic walls containing an ideal gas. The internal energy of this ideal gas is given by 1.5 nRT. The tube is divided into two equal parts by a fixed diathermic wall. Initially, the pressure and the temperature are p1, T1 on the left and p2, T2 on the right. The system is left for sufficient time so that the temperature becomes equal on the two sides. (a) How much work has been done by the gas on the left part? (b) Find the final pressures on the two sides. (c) Find the final equilibrium temperature. (d) How much heat has flown from the gas on the right to the gas on the left?
Which of the following system freely allows the exchange of energy and matter with its environment?
Define heat.
What is the internal energy of the system, when the amount of heat Q is added to the system and the system does not do any work during the process?
When does a system lose energy to its surroundings and its internal energy decreases?
A thermodynamic system goes from states
(i) P, V to 2P, V (ii) P, V to P, 2V
The work done in the two cases is ____________.
Figure shows the P-V diagram of an ideal gas undergoing a change of state from A to B. Four different parts I, II, III and IV as shown in the figure may lead to the same change of state.

- Change in internal energy is same in IV and III cases, but not in I and II.
- Change in internal energy is same in all the four cases.
- Work done is maximum in case I
- Work done is minimum in case II.
A person of mass 60 kg wants to lose 5kg by going up and down a 10 m high stairs. Assume he burns twice as much fat while going up than coming down. If 1 kg of fat is burnt on expending 7000 kilo calories, how many times must he go up and down to reduce his weight by 5 kg?
In thermodynamics, heat and work are ______.
An expansion process on a diatomic ideal gas (Cv = 5/2 R), has a linear path between the initial and final coordinates on a pV diagram. The coordinates of the initial state are: the pressure is 300 kPa, the volume is 0.08 m3 and the temperature is 390 K. The final pressure is 90 kPa and the final temperature s 320 K. The change in the internal energy of the gas, in SI units, is closest to:
If a gas is compressed adiabatically:
A system releases 125 kJ of heat while 104 kJ work is done on the system. Calculate the change in internal energy.
Explain the change in internal energy of a thermodynamic system (the gas) by heating it.
