Advertisements
Advertisements
प्रश्न
Methane molecule is non-polar molecule. Explain.
उत्तर
During the formation of a non-polar covalent bond between two similar atoms or dissimilar atoms, the atoms involved in sharing share the electrons equally. The molecule of methane has four carbon-hydrogen single covalent bonds. It is a non-polar covalent compound as the electrons are shared by the carbon and hydrogen atoms equally and hence the shared pair lies between the atoms at an equal distance from both carbon and hydrogen atom.
\[\begin{array}{cc}
\ce{H}\\
|\\
\ce{H - C - H}\\
|\\
\ce{H}\\
\end{array}\]
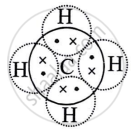 Methane
Methane
APPEARS IN
संबंधित प्रश्न
Explain why carbon forms compounds mainly by covalent bond.
State the reason to explain why covalent compounds "have low melting and boiling points."
Give one example, state what are covalent compounds?
The number of carbon atoms joined in a spherical molecule of buckminsterfullerene is:
(a) fifty
(b) sixty
(c) seventy
(d) ninety
Define a coordinate bond and give the conditions for its formation.
Give reason as to why hydrogen chloride can be termed as a polar covalent compound.
Element A has 2 electrons in its M shell. Element B has atomic number 7.
If the compound formed between A and B is melted and an electric current is passed through the molten compound, the element A will be obtained at the ______ and B at the ______ of the electrolytic cell.
Name two carbon compounds used in day-to-day life.
Acetic acid was added to a solid X kept in a test tube. A colourless, odourless gas Y was evolved. The gas was passed through lime water, which turned milky. It was concluded that ______.
`"CH"_3 - "CH"_2 - "OH" overset("Alkaline""KMnO"_4 + "Heat")(->) "CH"_3 - "COOH"`
In the above given reaction, alkaline KMnO4 acts as
