Advertisements
Advertisements
Question
Methane molecule is non-polar molecule. Explain.
Solution
During the formation of a non-polar covalent bond between two similar atoms or dissimilar atoms, the atoms involved in sharing share the electrons equally. The molecule of methane has four carbon-hydrogen single covalent bonds. It is a non-polar covalent compound as the electrons are shared by the carbon and hydrogen atoms equally and hence the shared pair lies between the atoms at an equal distance from both carbon and hydrogen atom.
\[\begin{array}{cc}
\ce{H}\\
|\\
\ce{H - C - H}\\
|\\
\ce{H}\\
\end{array}\]
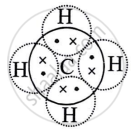 Methane
Methane
APPEARS IN
RELATED QUESTIONS
State the reason to explain why covalent compounds "are bad conductors of electricity".
Draw the electron-dot structure of CO2 compound and state the type of bonding.
Two non-metals combine with each other by the sharing of electrons to form a compound X.
(a) What type of chemical bond is present in X?
(b) State whether X will have a high melting point or low melting point.
(c) Will it be a good conductor of electricity or not?
(d) Will it dissolve in an organic solvent or not?
Explain the following term with example.
Unsaturated hydrocarbon
What do you understand by lone pair and shared pair?
An element L consists of molecules.
Why L is heated with iron metal, it forms a compound FeL. What chemical term would you use to describe the change undergone by L?
Electrons are getting added to an element Y:
which electrode will Y migrate to during the process of electrolysis?
Explain the following:
Polar covalent compounds conduct electricity?
Assertion (A): Melting point and boiling point of ethanol are lower than that of sodium chloride.
Reason (R): The forces of attraction between the molecules of ionic compounds are very strong.
Explain dipole (polar) molecule by taking hydrogen chloride as an example.
