Advertisements
Advertisements
प्रश्न
Name the reagent used in the following reaction:
Bromination of phenol to 2, 4, 6-tribromophenol.
उत्तर
Aqueous bromine, i.e., Br2/H2O, is used in the bromination of phenol to 2, 4, 6-tribromophenol.
APPEARS IN
संबंधित प्रश्न
Give simple chemical tests to distinguish between the following pairs of compounds: Benzoic acid and Phenol
Write the structures of A, B, C, D and E in the following reactions:
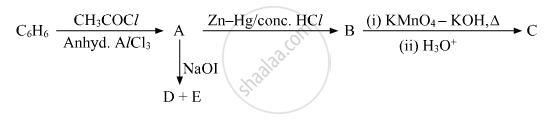
Write the equation involved in the following reaction:
Reimer-Tiemann reaction
Write the equation involved in the following reaction:
Kolbe’s reaction
While separating a mixture of ortho and para nitrophenols by steam distillation, name the isomer which will be steam volatile. Give reason.
Explain the following with an example.
Kolbe’s reaction.
When phenol is treated with excess bromine water, it gives:
On distilling phenol with Zn dust, one gets:
\[\ce{C2H5OH + SOCl2 ->[Pyridine] C2H5Cl + SO2 + HCl}\]
The above reaction is known as:
Which of the following reactions is used to prepare salicylaldehyde?
Phenol does not undergo nucleophilic substitution reaction easily due to ______.
Which of the following compounds is aromatic alcohol?
| (A) |  |
| (B) | 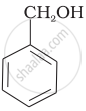 |
| (C) | 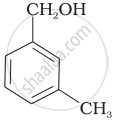 |
| (D) | 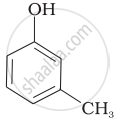 |
Which of the following are used to convert \[\ce{RCHO}\] into \[\ce{RCH2OH}\]?
(i) \[\ce{H2/Pd}\]
(ii) \[\ce{LiAlH4}\]
(iii) \[\ce{NaBH4}\]
(iv) Reaction with \[\ce{RMgX}\] followed by hydrolysis
Which of the following are benzylic alcohols?
(i) \[\ce{C6H5 - CH2 - CH2OH}\]
(ii) \[\ce{C6H5 - CH2OH}\]
(iii) \[\begin{array}{cc}
\ce{C6H5 - CH - OH}\\
\phantom{}|\phantom{.}\\
\phantom{..}\ce{CH3}\phantom{}
\end{array}\]
(iv) \[\begin{array}{cc}
\ce{C6H5 - CH2 - CH - OH}\\
\phantom{.......}|\phantom{}\\
\phantom{.........}\ce{CH3}\phantom{}
\end{array}\]
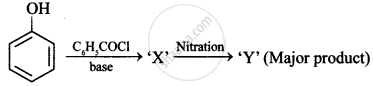
Nitration is an example of aromatic electrophilic substitution and its rate depends upon the group already present in the benzene ring. Out of benzene and phenol, which one is more easily nitrated and why?
Attacking species in nitration of benzene in presence of fuming HNO3 is
Write the equations for the following reaction:
Phenol is treated with chloroform in the presence of NaOH
