Advertisements
Advertisements
प्रश्न
Name two items that can serve as a model for Gay Lusaac’s law and explain.
उत्तर
- Firing a bullet: When gunpowder burns, it creates; a significant amount of superheated gas. The high pressure of the hot gas behind the bullet forces it out, of the barrel of the gun.
- Heating food in an oven: When you keep food in an oven for heating, the air inside the oven is heated, thus pressurized.
APPEARS IN
संबंधित प्रश्न
State (i) the three variables for gas laws and (ii) SI units of these variables.
Give reason for the following:
Gases have a lower density compared to solids or liquids.
Convert the following temperature from degree Celcius to kelvin.
−15° C
Convert 101.325 kPa to bar.
Identify the gas laws from the following diagram.
| Diagram | Gas laws |
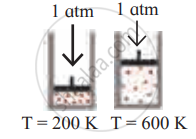 |
______________ |
Write the statement for Boyle’s law
State Boyle's law.
Volume of a balloon at 25°C and 1 bar pressure is 2.27 L. If the pressure of the gas in balloon is reduced to 0.227 bar, what is the rise in volume of a gas?
The volume of 400 cm3 chlorine gas at 400 mm of Hg is decreased to 200 cm3 at constant temperature. What is the new pressure of gas?
If 300 mL of a gas at 26.85°C is cooled to 6.85°C at constant pressure. What will be the final volume of gas?
