Advertisements
Advertisements
प्रश्न
Retention of configuration is observed in ______.
पर्याय
SN1 reaction
SN2 reaction
Neither SN1 nor SN2 reaction
SN2 reaction as well as SN1 reaction
उत्तर
Retention of configuration is observed in SN1 reaction.
Explanation:
Both retention and inverted products are generated in SN1 reactions, but only the inverted product is formed in SN2 reactions.
APPEARS IN
संबंधित प्रश्न
Discuss the mechanism of alkaline hydrolysis of bromomethane.
Given reasons: C–Cl bond length in chlorobenzene is shorter than C–Cl bond length in CH3–Cl.
Arrange the compounds of the following set in order of reactivity towards SN2 displacement:
1-Bromobutane, 1-Bromo-2, 2-dimethylpropane, 1-Bromo-2-methylbutane, 1-Bromo-3-methylbutane
Which of the following is optically inactive?
The process of separation of a racemic modification into d and l-enantiomers is called ____________.
Complete the following analogy:
Same molecular formula but different structures: A : : Non superimposable mirror images: B
How do polar solvents help in the first step in SN1 mechanism?
Match the reactions given in Column I with the types of reactions given in Column II.
| Column I | Column II | |
| (i) | 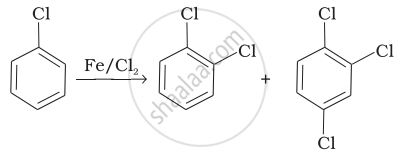 |
(a) Nucleophilic aromatic substitution |
| (ii) | \[\begin{array}{cc} \ce{CH3 - CH = CH2 + HBr -> CH3 - CH - CH3}\\ \phantom{............................}|\phantom{}\\ \phantom{.............................}\ce{Br}\phantom{} \end{array}\] |
(b) Electrophilic aromatic substitution |
| (iii) | 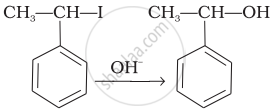 |
(c) Saytzeff elimination |
| (iv) | 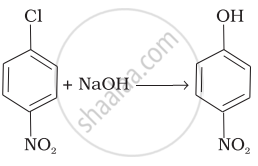 |
(d) Electrophilic addition |
| (v) | \[\begin{array}{cc} \ce{CH3 CH2 CH CH3 ->[alc.KOH] CH3 CH = CH CH3}\\ \phantom{}|\phantom{..........................}\\ \phantom{}\ce{Br}\phantom{........................} \end{array}\] |
(e) Nucleophilic substitution (SN1) |
Give reason for the following:
The product formed during SN1 reaction is a racemic mixture.
Convert bromoethane to propanamine.
