Advertisements
Advertisements
प्रश्न
Discuss the mechanism of alkaline hydrolysis of bromomethane.
उत्तर
a. Alkaline hydrolysis of bromomethane follows bimolecular nucleophilic substitution (SN2)mechanism. The hydrolysis reaction can be written as follows:

1. Approach of the nucleophile (Backside attack):
i. The nucleophile (OH-) slowly approaches the carbon atom from the opposite side of the C - Br bond.
ii. C – OH weak bond is formed, while the existing C – Br bond gradually weakens.
iii. It is a slow process and hence, it is the rate determining step (R.D.S.).
2. Transition state (Activated complex): With the approach of OH- group and the
gradual departure of Br, a stage comes where the central atom is attached to five substituents. This state is known as transition state of reaction.
At this stage, the three hydrogen atoms lie in a plane perpendicular to the HO – C – Br axis.
3. Stereochemistry of SN2 reaction:
The attack might take place from back as well as from front side.
i. If backside attack takes place:
As shown in the figure given below, the OH group occupies a position in the product which is opposite to the position of Br. Similarly the positions of H2 and
H3 in the reactant and in the product are on opposite sides i.e., inverted due to the back side attack. This is known as inversion of configuration. Thus, backside
attack of nucleophile leads to inversion of configuration.

ii. If front side attack takes place: In this situation, the OH- occupies the same position which was occupied by Br in the reactant and the position of H1, H2 and
H3 also remain the same. Therefore, the configuration of the carbon is retained. This is known as retention of configuration.

The product X is obtained with inversion of configuration and not Y, with retention of configuration (X and Y are enantiomers). Thus, in SN2 reaction, the nucleophile attacks from backside leading to the inversion of configuration.
APPEARS IN
संबंधित प्रश्न
How do you convert the following:
Ethanol to propanenitrile
Given reasons: C–Cl bond length in chlorobenzene is shorter than C–Cl bond length in CH3–Cl.
Which would undergo SN2 reaction faster in the following pair and why ?
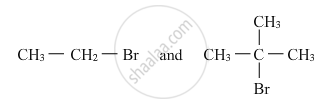
Write the structures of A, B and C in the following:

In the following pair of halogen compounds, which compound undergoes a faster SN1 reaction?
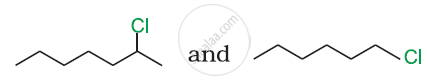
Which compound in the following pair will react faster in SN2 reaction with OH−?
CH3Br or CH3I
Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH2CH2OH + SOCl2 ->}\]
Write the mechanism of the following reaction:
\[\ce{{n}BuBr + KCN ->[EtOH-H2O] {n}BuCN}\]
Arrange the compounds of the following set in order of reactivity towards SN2 displacement:
1-Bromobutane, 1-Bromo-2, 2-dimethylpropane, 1-Bromo-2-methylbutane, 1-Bromo-3-methylbutane
Which would undergo SN2 reaction faster in the following pair and why ?
CH3 – CH2 – Br and CH3 – CH2 – I
What is the action of the following on ethyl bromide?
moist silver oxide
Answer the following question.
Write one stereochemical difference between SN1 and SN2 reactions.
Which of the following is an example of SN2 reaction?
In the SN1 reaction, racemization takes place. It is due to:
Isopropyl chloride undergoes hydrolysis by:
Which of the following is the correct order of decreasing SN2 reactivity?
The process of separation of a racemic modification into d and l-enantiomers is called ____________.
Among the following, the dissociation constant is highest for:
The increasing order of nucleophilicity would be:
The increasing order of reactivity towards SN1 mechanism is:
(I) \[\begin{array}{cc}
\ce{CH3-CH-CH2-CH3}\\
|\phantom{........}\\
\ce{CH3}\phantom{.....}
\end{array}\]
(II) CH3CH2CH2Cl
(III) P–CH3O–C6H4–CH2Cl
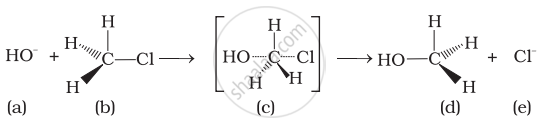
Which of the statements are correct about above reaction?
(i) (a) and (e) both are nucleophiles.
(ii) In (c) carbon atom is sp3 hybridised.
(iii) In (c) carbon atom is sp2 hybridised.
(iv) (a) and (e) both are electrophiles.
How do polar solvents help in the first step in SN1 mechanism?
Racemisation occurs in ______.
Give the mechanism of the following reaction:
\[\ce{CH3CH2OH ->[H2SO4][413 K] CH3CH2-O-CH2CH3 + H2O}\]
An organic compound A with the molecular formula (+) C4H9Br undergoes hydrolysis to form (+) C4H9OH. Give the structure of A and write the mechanism of the reaction.
Identify the product in the following reaction: 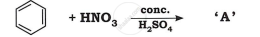
Acetic anhydride from acetic acid
Discuss the mechanism of alkaline hydrolysis of methyl bromide.
