Advertisements
Advertisements
प्रश्न
The compound that is not an ore of aluminum is ______.
पर्याय
Cryolite
Corundum
Fluorspar
Bauxite
उत्तर
The compound that is not an ore of aluminium is fluorspar.
Explanation:
Cryolite is Na3AlF6, corundum is Al2C3, fluorspar is CaF2 and bauxite is Al2C3.2H2O.
APPEARS IN
संबंधित प्रश्न
For the substance given below, describe the role played in the extraction of aluminium.
Cryolite
For cryolite, explain its significance in the extraction of aluminium.
In order to obtain 1 tonne of aluminium, the following inputs are required: 4 tonnes of bauxite, 150 kg of sodium hydroxide and 600 kg of graphite. The aluminium compound in bauxite is aluminium oxide and the main impurity is iron (III) oxide. Aluminium is obtained by the electrolysis of aluminium oxide dissolved in cryolite.
When bauxite is treated with sodium hydroxide solution, what happens to:
- the aluminium oxide,
- the iron (III) oxide?
Give reason for the following:
Nitric acid can be stored in aluminium containers.
In order to obtain one tone of aluminium, the following inputs are required:
4 tones of bauxite, 150 Kg of sodium hydroxide and 600 Kg of graphite.
The aluminium compound in bauxite is aluminium oxide and the main impurity is iron (II) oxide. Aluminium is obtained by the electrolysis of aluminium oxide dissolved in cryolite.
(i) Name the process used for purification of bauxite.
(ii) Write the equation to show the action of heat on aluminium hydroxide.
In order to obtain one tone of aluminium, the following inputs are required:
4 tones of bauxite, 150 Kg of sodium hydroxide and 600 Kg of graphite.
The aluminium compound in bauxite is aluminium oxide and the main impurity is iron (II) oxide. Aluminium is obtained by the electrolysis of aluminium oxide dissolved in cryolite.
In construction work, why is the alloy of aluminium, duralumin used rather than pure aluminium.
Name the following:
The middle region of the blast furnace.
Correct the following statement :
Haematite is the chief ore of aluminium.
Name the alloy used for the following purpose.
Surgical instruments
The following sketch illustrates the process of conversion of Alumina to Aluminium:
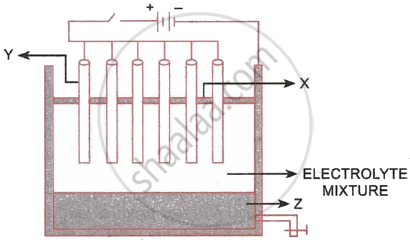
- Name the constituent of the electrolyte mixture which has a divalent metal in it.
- Name the powdered substances ‘X’ sprinkled on the surface of the electrolyte mixture.
- What is the name of the process?
- Write the reactions taking place at the electrodes ‘Y’ (anode) and ‘Z’ (cathode), respectively.
