Advertisements
Advertisements
प्रश्न
The conductivity of 0.20 mol L−1 solution of KCl is 2.48 × 10−2 S cm−1. Calculate its molar conductivity and degree of dissociation (α). Given λ0 (K+) = 73.5 S cm2 mol−1 and λ0 (C1−) = 76.5 S cm2 mol−1.
उत्तर
Conductivity of KCl solution = 2.48×10–2 S cm–1
Concentration of KCl solution = 0.20 mol L–1
= 0.20 × 1000 mol cm–3
= 200 mol cm–3
Molar conductivity
`Lambda_m=k/c`
`=(2.48xx10^(-2)" S "cm^(-1))/(200" mol "cm^(-3))`
`=124xx10^(-6)" S "cm^2" mol"^(-1)`
Given:
`lambda_(K^+)^@=73.5" S "cm^2" mol"^(-1)`
`lambda_(Cl^-)^@=76.5" S "cm^2" mol"^(-1)`
Degree of dissociation
`alpha=Lambda_m/Lambda_m^@" "" "" "" ".......("i")`
`Lambda_(m(KCl))^@=lambda_(K+)^@+lambda_(Cl^-)^@`
`=73.5+76.5`
`=150" S "cm^2" mol"^(-1)`
`"Substituting the values of "Lambda_m" and "Lambda_(m(KCl))^@" in (i), we get"`
`alpha=(124xx10^(-6)" S "cm^2" mol"^(-1))/(150" S "cm^2" mol"^(-1))=0.82xx10^(-6)`
संबंधित प्रश्न
Define "Molar conductivity".
Define limiting molar conductivity.
State Kohlrausch law of independent migration of ions.
The S.I. unit of cell constant for conductivity cell is __________.
In the plot of molar conductivity (∧m) vs square root of concentration (c1/2), following curves are obtained for two electrolytes A and B:
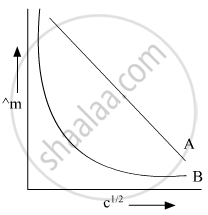
Answer the following:
(i) Predict the nature of electrolytes A and B.
(ii) What happens on extrapolation of ∧m to concentration approaching zero for electrolytes A and B?
Solutions of two electrolytes ‘A’ and ‘B’ are diluted. The Λm of ‘B’ increases 1.5 times while that of A increases 25 times. Which of the two is a strong electrolyte? Justify your answer.
Why on dilution the m Λm of \[\ce{CH3COOH}\] increases very fast, while that of \[\ce{CH3COONa}\] increases gradually?
Assertion: Copper sulphate can be stored in zinc vessel.
Reason: Zinc is less reactive than copper.
Which of the following halogen acids is the strongest reducing agent?
The solubility of Co2[Fe(CN)6] in water at 25°C from the following data:
Conductivity of saturated solution of Co2[Fe(CN)6] = 2.06 × 10−6 ohm−1 cm−1 and that of water = 4.1 × 10−7 ohm−1 cm−1. The ionic molar conductivities of Co2+ and [Fe(CN)6]4− are 86 and 444 ohm−1 cm2 mol−1 respectively, is ______ × 10−6 mol/L.
