Advertisements
Advertisements
प्रश्न
The time required for 10% completion of a first order reaction at 298 K is equal to that required for its 25% completion at 308 K. If the value of A is 4 × 1010 s−1. Calculate k at 318 K and Ea.
उत्तर
t = `2.303/"k"_1 log ["R"]_0/(90/100 ["R"]_0)`
t = `2.303/"k"_2 log ["R"]_0/(75/100 ["R"]_0)`
t = `2.303/"k"_1 log 10/9`,
t = `2.303/"k"_2 log 4/3`
`2.303/"k"_1 log 10/9 = 2.303/"k"_2 log 4/3`
`=> "k"_2/"k"_1 = (log(4/3))/(log(10/9))`
= `log 1.333/log 1.111`
= `0.1249/0.0457`
= 2.733
log`"k"_2/"k"_1 = E_a/(2.303"R")(("T"_2 - "T"_1)/("T"_1"T"_2))`
⇒ log 2.733 = `E_a/(2.303 xx 8.314) ((308 - 298)/(298 xx 308))`
Ea = `(2.303 xx 8.314 xx 308 xx 298)/10 xx 0.4367`
= `(19.147 xx 308 xx 298)/10 xx 0.4367`
= 76.75 kJ mol−1
ln k = ln A `- E_a/("RT")`
log k = log A `- E_a/(2.303 "RT")`
= log (4 × 1010) `- (76.75 xx 1000)/(2.303 xx 8.314 xx 318)`
= 10.6021 `- 76750/6088.791`
= 10.6021 − 12.6051
= −2.003
k = Antilog (−2.003) = 9.93 × 10−3
APPEARS IN
संबंधित प्रश्न
The following data were obtained during the first order thermal decomposition of SO2Cl2 at a constant volume.
\[\ce{SO2Cl2_{(g)} -> SO2_{(g)} + Cl2_{(g)}}\]
| Experiment | Time/s–1 | Total pressure/atm |
| 1 | 0 | 0.5 |
| 2 | 100 | 0.6 |
Calculate the rate of the reaction when total pressure is 0.65 atm.
In a pseudo first order hydrolysis of ester in water, the following results were obtained:
| t/s | 0 | 30 | 60 | 90 |
| [A]/mol L−1 | 0.55 | 0.31 | 0.17 | 0.085 |
Calculate the average rate of reaction between the time interval 30 to 60 seconds.
The rate constant for a first order reaction is 60 s−1. How much time will it take to reduce the initial concentration of the reactant to its `1/16`th value?
Define order of reaction. How does order of a reaction differ from molecularity for a complex reaction?
A first order reaction is 50% complete in 25 minutes. Calculate the time for 80% completion of the reaction.
Show that the time required for 99.9% completion of a first-order reaction is three times the time required for 90% completion.
Which of the following graphs is correct for a first order reaction?

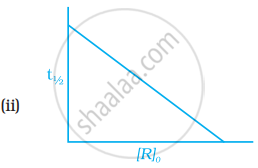
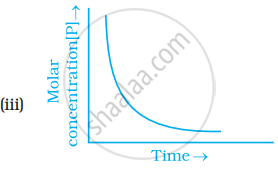
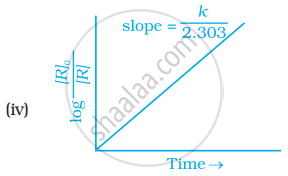
With the help of an example explain what is meant by pseudo first order reaction.
In the presence of acid, the initial concentration of cane sugar was reduced from 0.2 M to 0.1 Min 5 hours and to 0.05 Min 10 hours. The reaction must be of?
A first order reaction is 50% complete in 20 minute What is rate constant?
In the first order reaction, half of the reaction is complete in 100 seconds. The time for 99% of the reaction to occurs will be
In a first order reaction the concentration of reactants decreases from 400mol L-1 to 25 mol L-1 in 200 seconds. The rate constant for the reaction is ______.
The slope in the plot of `log ["R"]_0/(["R"])` Vs. time for a first-order reaction is ______.
How will you represent first order reactions graphically?
The following data were obtained during the decomposition of SO2Cl2 at the constant volume. SO2Cl2 →SO2(g) + Cl2(g)
| Time (s) | Total Pressure (bar) |
| 0 | 0.5 |
| 100 | 0.6 |
Calculate the rate constant of the reaction.
Show that `t_(1/2)= 0.693/k` for first reaction.
Write the unit of rate constant [k] for the first order reaction.
