Advertisements
Advertisements
प्रश्न
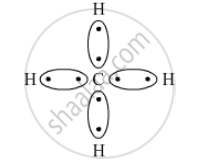
What type of bonds are present in CH4 molecule? Draw their electron-dot structures.
उत्तर
Covalent bond is the chemical bond present in CH4 as a carbon atom shares its electrons with four hydrogen atoms.
The electron-dot structure is:
APPEARS IN
संबंधित प्रश्न
What type of chemical bond is formed between carbon and bromine?
What type of bonds are present in CO2 molecule? Draw their electron-dot structures.
How buckminsterfullerene is it related to diamond and graphite?
How will you find out which of the water soluble compound A or B is ionic?
Describe the structure of graphite with the help of a labelled diagram.
Give the formulae of the chlorides of the elements X and Y having atomic numbers of 3 and 6 respectively. Will the properties of the two chlorides be similar or different? Explain your answer.
Draw all possile structural formulae of compound from their molecular formula given below.
C4H10
Explain the following briefly:
Sodium chloride dissolves in water but carbon tetra chloride is insoluble in water.
(a) Compound X consists of molecules.
Choose the letter corresponding to the correct answer from the choices (a), (b), (c) and (d) given below
In the liquid state, X will
The electronic configuration of N2 is 2, 5. How many electrons in the outer shell of a N atom are not involved in the formation of a nitrogen molecule?
State the type of bond formed, and draw Lewis structure of water.
In the formation of electrovalent compounds, electrons are transferred from one element to another. How are electrons involved in the formation of a covalent compound?
Name the types of Hydrocarbons.
Write the molecular formula of the given compound.
Propylene
Write scientific reason.
Carbon has the property of forming many compounds.
Two carbon atoms can always form one or two covalent bonds.
"Carbon prefers to share its valence electrons with other atoms of carbon or with atoms of other elements rather than gaining or losing the valence electrons in order to attain noble gas configuration." Give reasons to justify this statement.
Non-polar covalent compounds are ______ conductors of heat and electricity.
The number of single and double bonds present in a molecule of benzene (C6H6) respectively, are ______.
