Advertisements
Advertisements
प्रश्न
Write the equation for the reaction which takes place in question(a).
उत्तर
\[\ce{\underset{\text{Sodium Chloride}}{NaCl} + \underset{\text{Sulphuric acid(Conc.)}}{H2SO4} ->[<200°C] \underset{text{Sodium hydrogen sulphate}}{NaHSO4} + \underset{\text{Hydrogen chloride gas}}{HCl}}\]
APPEARS IN
संबंधित प्रश्न
Identify the gas evolved and give the chemical test in the following cases
Dilute hydrochloric acid reacts with sodium sulphite.
Identify the gas evolved and give the chemical test in the following cases
Dilute hydrochloric acid reacts with iron (II) sulphide.
The following are pertaining to the laboratory preparation of hydrogen chloride gas.
Write the equation for its preparation mentioning the condition required.
Give the balanced equation for the laboratory preparation of hydrogen chloride gas reaction.
The given set up in the figure is for the preparation of an acid.
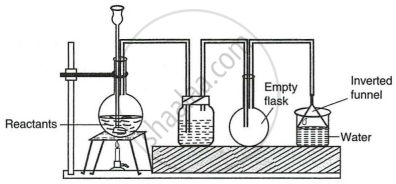
- Name the acid prepared by this method.
- Name the reactants used.
- Why an empty flask is used?
- What is the drying agent used? Why is this drying agent chosen?
- What is the role of the inverted funnel in the arrangement?
How will you prove that the gas prepared is HCI?
Describe an experiment to prove the following:
HCI gas contains the element chlorine.
Mention the reaction condition and give balanced equation to obtain : HCl gas from common salt
Hydrogen chloride gas, being highly soluble in water, is dried by ______.
State the observation for action of dilute hydrochloiric acid or iron (II) sulphate.
One chemical test that would enable you to distinguish between the following pair of chemicals. Describe what happens with each chemical or state 'no visible reaction'.
Sodium chloride solution and sodium nitrate solution.
One chemical test that would enable you to distinguish between the following pair of chemicals. Describe what happens with each chemical or state 'no visible reaction'.
Sodium chloride solution and sodium nitrate solution.
One chemical test that would enable you to distinguish between the following pair of chemicals. Describe what happens with each chemical or state 'no visible reaction'.
Sodium sulphate solution and sodium chloride solution.
Give one test to distinguish between the following pair of chemicals.
Iron (III) Chloride solution and copper chloride solution.
Answer the following question related to the laboratory preparation of the hydrogen chloride gas:
Write the chemical equation.
State a relevant reason for the following:
Hydrogen chloride gas cannot be dried over quick lime.
Complete and balance the following reaction, state whether dilute or conc. acid is used.
\[\ce{NH4OH + HCl ->}\]
Complete and balance the following reaction, state whether dilute or conc. acid is used.
\[\ce{NH4OH + HCl ->}\]
