Advertisements
Advertisements
Question
A compound 'A' of molecular formula C2H3OCl undergoes a series of reactions as shown below. Write the structures of A, B, C and D in the following reactions :
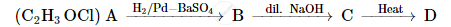
Solution
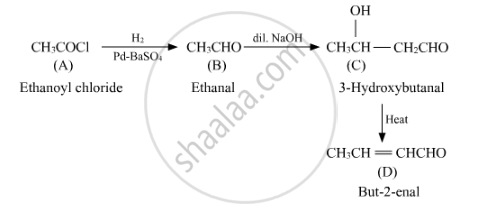
Using the given molecular formula, compound A is Ethanoyl Chloride CH3COCl, which undergoes reaction with poisoned palladium.
On carrying hydrogenation of A in the presence of poisoned palladium, we get an aldehyde. Hence, B can be Ethanal, CH3CHO.
An aldehyde, on treating with dilute alkali, undergoes aldol condensation reaction. Hence, C can be CH3CH(OH)CH2CHO.
On heating an aldol product, it loses water to produce a double bond and we get CH3CH=CHCHO.
Hence, we have
RELATED QUESTIONS
How will you convert ethanal into the following compound?
Butane-1, 3-diol
How will you convert ethanal into the following compound?
But-2-enal
Write structural formulas and names of four possible aldol condensation products from propanal and butanal. In each case, indicate which aldehyde acts as nucleophile and which as electrophile.
How will you bring about the following conversion in not more than two steps?
Benzaldehyde to 3-Phenylpropan-1-ol
Write chemical equations of the following reaction :
Propanone is treated with dilute Ba (OH)2-.
What is substituted imine called?
Which product is formed when the compound 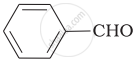 is treated with concentrated aqueous \[\ce{KOH}\] solution?
is treated with concentrated aqueous \[\ce{KOH}\] solution?
The major product of the following reaction is:
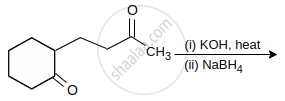

Identify A and B:
When acetaldehyde is treated with dilute NaOH, the following reaction is observed.
\[\begin{array}{cc}
\ce{2CH3 - CHO ->[dil.NaOH] CH3 - CH - CH2 - CHO}\\
\phantom{...............}|\\
\phantom{.................}\ce{OH}
\end{array}\]
- What are the functional groups in the product?
- Can another product be formed during the same reaction? (Deduce the answer by doing atomic audit of reactant and product).
- Is this an addition reaction or condensation reaction?
