Advertisements
Advertisements
Question
How will you convert ethanal into the following compound?
Butane-1, 3-diol
Solution
\[\ce{\underset{Ethanal}{2CH3CHO}->[Dil.NaOH][Aldol condensation]\underset{3-Hydroxybutanal}{\overset{4}{C}H3\overset{3}{C}HOH - \overset{2}{C}H2 - \overset{1}{C}HO}->[NaBH4][(Reduction)]\underset{Butane-1, 3-diol}{\overset{4}{C}H3 - \overset{3}{C}HOH - \overset{2}{C}H2 - \overset{1}{C}H2OH}}\]
APPEARS IN
RELATED QUESTIONS
How will you bring about the following conversion?
Ethanal to but-2-enal
Describe the following:
Cross aldol condensation
Give reasons Acetylation of aniline reduces its activation effect.
What is substituted imine called?
Explain aldol condensation reaction in detail.
What product will be formed on reaction of propanal with 2-methylpropanal in the presence of \[\ce{NaOH}\]? What products will be formed? Write the name of the reaction also.
Compound ‘A’ was prepared by oxidation of compound ‘B’ with alkaline \[\ce{KMnO4}\]. Compound ‘A’ on reduction with lithium aluminium hydride gets converted back to compound ‘B’. When compound ‘A’ is heated with compound B in the presence of \[\ce{H2SO4}\] it produces fruity smell of compound C to which family the compounds ‘A’, ‘B’ and ‘C’ belong to?
Why are carboxylic acids more acidic than alcohols or phenols although all of them have hydrogen atom attached to a oxygen atom \[\ce{(-O-H)}\]?
Assertion: The α-hydrogen atom in carbonyl compounds is less acidic.
Reason: The anion formed after the loss of α-hydrogen atom is resonance stabilised.
Give reasons to support the answer:
Presence of Alpha hydrogen in aldehydes and ketones is essential for aldol condensation.
Cross aldol condensation occurs between
Which of the following gives aldol con~ensation reaction?
Identify A and B from the following reaction:
\[\begin{array}{cc}
\ce{CH3}\phantom{.................}\\
|\phantom{....................}\\
\phantom{}\ce{2CH3 - C = O ->[Ba(OH)2] A ->[Δ] B + H2O}
\end{array}\]
Convert the following:
Acetaldehyde to But-2-enal
The major product of the following reaction is:
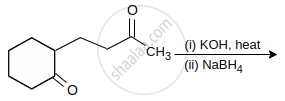
Assertion (A): The final product in Aldol condensation is always α, β-unsaturated carbonyl compound.
Reason (R): α, β-unsaturated carbonyl compounds are stabilised due to conjugation.
What is aldol condensation? Explain it with suitable examples.
