Advertisements
Advertisements
Question
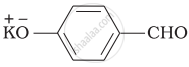
Which product is formed when the compound 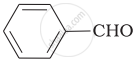 is treated with concentrated aqueous \[\ce{KOH}\] solution?
is treated with concentrated aqueous \[\ce{KOH}\] solution?
Options
Solution
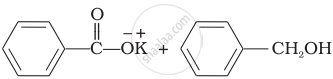
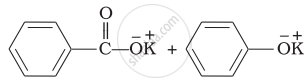
Explanation:
Benzaldehyde is having no a hydrogen. So, on reaction with aqueous KOH solution, it undergoes Cannizzaro’s reaction. One molecule of aldehyde is reduced and other is

APPEARS IN
RELATED QUESTIONS
What is meant by the following term? Give an example of the reaction in the following case.
Aldol
Write structural formulas and names of four possible aldol condensation products from propanal and butanal. In each case, indicate which aldehyde acts as nucleophile and which as electrophile.
Describe the following:
Cross aldol condensation
Write chemical equations of the following reaction :
Benzoyl chloride is hydrogenated in the presence of `"Pd"/(BaSO_4)`
Explain aldol condensation reaction in detail.
Compounds A and C in the following reaction are:
\[\ce{CH3CHO ->[(i) CH3MgBr][(ii) H2O] (A) ->[H2SO4, Δ] (B) ->[Hydroboration oxidation] (C)}\]
Why is there a large difference in the boiling points of butanal and butan-1-ol?
Compound ‘A’ was prepared by oxidation of compound ‘B’ with alkaline \[\ce{KMnO4}\]. Compound ‘A’ on reduction with lithium aluminium hydride gets converted back to compound ‘B’. When compound ‘A’ is heated with compound B in the presence of \[\ce{H2SO4}\] it produces fruity smell of compound C to which family the compounds ‘A’, ‘B’ and ‘C’ belong to?
Assertion: The α-hydrogen atom in carbonyl compounds is less acidic.
Reason: The anion formed after the loss of α-hydrogen atom is resonance stabilised.
What is aldol condensation? Explain it with suitable examples.