Advertisements
Advertisements
Question
Compounds A and C in the following reaction are:
\[\ce{CH3CHO ->[(i) CH3MgBr][(ii) H2O] (A) ->[H2SO4, Δ] (B) ->[Hydroboration oxidation] (C)}\]
Options
identical
positional isomers
functional isomers
optical isomers
Solution
positional isomers
Explanation:
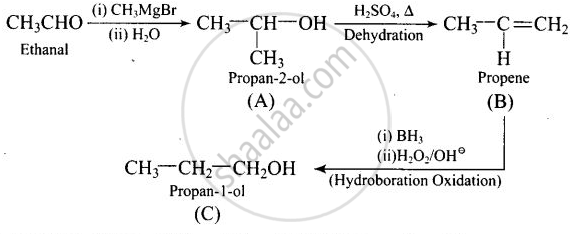
Thus, \[\begin{array}{cc}
\ce{CH3CH - OH}\\
|\phantom{....}\\
\ce{CH3}\phantom{..}
\end{array}\] and \[\ce{CH3 - CH2 - CH2OH}\] are positional isomers.
APPEARS IN
RELATED QUESTIONS
Write the products formed when CH3CHO reacts with the following reagents: CH3CHO in the presence of dilute NaOH
Why is alpha (α) hydrogen of carbonyl compounds acidic in nature?
Write chemical equations of the following reaction :
Benzoyl chloride is hydrogenated in the presence of `"Pd"/(BaSO_4)`
What is substituted imine called?
Cannizaro’s reaction is not given by ______.
Which product is formed when the compound 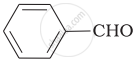 is treated with concentrated aqueous \[\ce{KOH}\] solution?
is treated with concentrated aqueous \[\ce{KOH}\] solution?
What product will be formed on reaction of propanal with 2-methylpropanal in the presence of \[\ce{NaOH}\]? What products will be formed? Write the name of the reaction also.
Why are carboxylic acids more acidic than alcohols or phenols although all of them have hydrogen atom attached to a oxygen atom \[\ce{(-O-H)}\]?
Identify A and B from the following reaction:
\[\begin{array}{cc}
\ce{CH3}\phantom{.................}\\
|\phantom{....................}\\
\phantom{}\ce{2CH3 - C = O ->[Ba(OH)2] A ->[Δ] B + H2O}
\end{array}\]
\[\ce{CH3-CH2-CHO ->[dil][alkali] Product}\]
The product in the above reaction is:
