Advertisements
Advertisements
Question
Cannizaro’s reaction is not given by ______.
Options
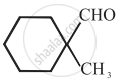
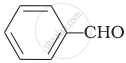
\[\ce{HCHO}\]
\[\ce{CH3CHO}\]
Solution
Cannizaro’s reaction is not given by \[\ce{CH3CHO}\].
Explanation:
\[\ce{CH3CHO}\] will not give Cannizzaro’s reaction because it contains a-hydrogen while other three compounds have no a-hydrogen. Hence, they will give Cannizzaro’s reaction.
APPEARS IN
RELATED QUESTIONS
How will you bring about the following conversion?
Ethanal to but-2-enal
Write structural formulas and names of four possible aldol condensation products from propanal and butanal. In each case, indicate which aldehyde acts as nucleophile and which as electrophile.
Give reasons Acetylation of aniline reduces its activation effect.
Compounds A and C in the following reaction are:
\[\ce{CH3CHO ->[(i) CH3MgBr][(ii) H2O] (A) ->[H2SO4, Δ] (B) ->[Hydroboration oxidation] (C)}\]
Which of the following conversions can be carried out by Clemmensen Reduction?
(i) Benzaldehyde into benzyl alcohol
(ii) Cyclohexanone into cyclohexane
(iii) Benzoyl chloride into benzaldehyde
(iv) Benzophenone into diphenyl methane
Why is there a large difference in the boiling points of butanal and butan-1-ol?
Why are carboxylic acids more acidic than alcohols or phenols although all of them have hydrogen atom attached to a oxygen atom \[\ce{(-O-H)}\]?
Predict the reagent for carrying out the following transformations:
Ethanal to 3-hydroxy butanal
Why is the α-hydrogens of aldehydes and ketones are acidic in nature?
Assertion (A): The final product in Aldol condensation is always α, β-unsaturated carbonyl compound.
Reason (R): α, β-unsaturated carbonyl compounds are stabilised due to conjugation.
