Advertisements
Advertisements
Question
A gas contained in a cylinder surrounded by a thick layer of insulating material is quickly compressed has there been a transfer of heat?
Options
Yes
No
Solution
No.
There is no transfer of heat energy, as the cylindrical vessel is surrounded by an insulating material, which doesn't allow heat transfer.
APPEARS IN
RELATED QUESTIONS
Answer in brief:
A gas contained in a cylinder surrounded by a thick layer of insulating material is quickly compressed has work been done?
Answer in brief.
What sets the limits on the efficiency of a heat engine?
Draw a p-V diagram and explain the concept of positive and negative work. Give one example each.
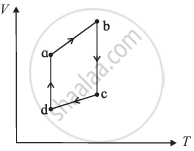
The figure shows the V-T diagram for one cycle of a hypothetical heat engine which uses the ideal gas. Draw the p-V diagram diagram of the system.
The efficiency of a heat engine working between the freezing point and boiling point of water is ____________.
State Clausius form of the second law of thermodynamics.
State Kelvin-Planck's statement of the second law of thermodynamics.
Define heat engine.
What are the processes involves in a Carnot engine?
State the second law of thermodynamics in terms of entropy.
Why does heat flow from a hot object to a cold object?
Explain the heat engine and obtain its efficiency.
Explain in detail the Carnot heat engine.
Derive the expression for Carnot engine efficiency.
Explain the second law of thermodynamics in terms of entropy.
Suppose a person wants to increase the efficiency of the reversible heat engine that is operating between 100°C and 300°C. He had two ways to increase efficiency.
- By decreasing the cold reservoir temperature from 100°C to 50°C and keeping the hot reservoir temperature constant
- by increasing the temperature of the hot reservoir from 300°C to 350°C by keeping the cold reservoir temperature constant.
Which is the suitable method?
A Carnot engine whose efficiency is 45% takes heat from a source maintained at a temperature of 327°C. To have an engine of efficiency of 60% what must be the intake temperature for the same exhaust (sink) temperature?
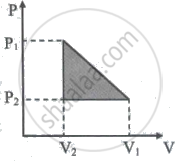
10 One mole of a van der Waals' gas obeying the equation `("P" + "a"/"V"^2)`(V - b) = RT undergoes the quasi-static cyclic process which is shown in the P-V diagram. The net heat absorbed by the gas in this process is ______
For a heat engine operating between temperatures t1 °C and t2 °C, its efficiency will be ______.
Heat engine transfers ______.
A heat engine operates between a cold reservoir at temperature T2 = 400 K and a hot reservoir at temperature T1. It takes 300 J of heat from the hot re ervoir and delivers 240 J of heat to the cold reservoir in a cycle. The minimum temperature of the hot reservoir has to be ______ K.
Which statement is incorrect?
Let η1 is the efficiency of an engine at T1 = 447°C and T2 = 147°C while η2, is the efficiency at T1 = 947°C and T2 = 47°C. The ratio `eta_1/eta_2` will be ______.
Draw a neat labelled P - V diagram for a typical heat engine.
The thermal efficiency of a heat engine is 25%. If in one cycle the heat absorbed from the hot reservoir is 50000 J, what is the heat rejected to the cold reservoir in one cycle?
What does a heat engine consist of?
What is a heat engine?
Mention any two elements of a heat engine.
