Advertisements
Advertisements
Question
A solution reacts with zinc granules to give a gas which burns with a 'pop' sound. The solution contains:
(a) Mg(OH)2
(b) Na2CO3
(c) NaCl
(d) HCl
Solution
HCl
Zinc granules react with HCl to give hydrogen gas, which burns with a ‘pop’ sound.
APPEARS IN
RELATED QUESTIONS
10 mL of a solution of NaOH is found to be completely neutralised by 8 mL of a given solution of HCl. If we take 20 mL of the same solution of NaOH, the amount of HCl solution (the same solution as before) required to neutralise it will be:
What happens when an acid reacts with a metal?
Write a balanced chemical equation of the reaction which takes place.
Explain the following:
Lead carbonate does not react with dilute HCl.
Answer the following question:
What is the universal indicator? Does Mg (OH)2 react with sodium hydroxide? If not, why?
A solution of NaCl
(i) will turn red litmus blue
(ii) will turn pH paper green
(iii) will turn blue litmus red
(iv) will not affect litmus
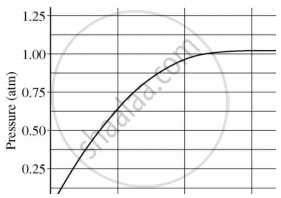
A student added 10 g of calcium carbonate in a rigid container, secured it tightly and started to heat it. After some time, an increase in pressure was observed, the pressure reading was then noted at intervals of 5 mins and plotted against time, in a graph as shown below. During which time interval did maximum decomposition take place?
Name the acid present in ant sting and give its chemical formula. Also give the common method to get relief from the discomfort caused by the ant sting.
What happens when nitric acid is added to egg shell?
Are all acids corrosive in nature? Name a few acids which are non-corrosive and may be part of our food.
