Advertisements
Advertisements
Question
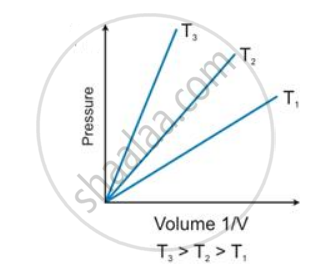
According to Boyle’s law, the shape of the graph between pressure and reciprocal of volume is _______.
Solution
According to Boyle’s law, the shape of the graph between pressure and reciprocal of volume is Straight line.
APPEARS IN
RELATED QUESTIONS
State the law which is represented by the following graph:
Give reasons for the following:
Inflating a balloon seems to violate Boyle's law.
A certain amount of a gas occupies a volume of 0.4 litre at 17°C. To what temperature should it be heated so that its volume gets (a) doubled, (b) reduced to half, pressure remaining constant?
At 0°C and 760 mmHg pressure, a gas occupies a volume of 100 cm3. Kelvin temperature of the gas is increased by one-fifth and the pressure is increased one and a half times. Calculate the final volume of the gas.
A certain mass of a gas occupies 2 litres at 27°C and 100 Pa. Find the temperature when volume and pressure become half of their initial values.
Calculate the volume occupied by 2 g of hydrogen at 27°C and 4-atmosphere pressure if at STP it occupies 22.4 litres.
Calculate the volume of dry air at STP that occupies 28 cm3 at 14°C and 750 mmHg pressure when saturated with water vapour. The vapour pressure of water at 14°C is 12 mmHg.
Assuming temperature remaining constant calculate the pressure of the gas in the following:
The pressure of a gas having volume 1000 cc. originally occupying 1500 cc. at 720 mm. pressure.
Fill in the blank with the correct word, from the words in option:
If the pressure of a fixed mass of a gas is kept constant and the temperature is increased, the volume correspondingly _______
State-the law of volume
