Advertisements
Advertisements
Question
An element X has a valency of 2. Write the simplest formula for oxide of the element.
Solution
Valency of X = 2
Valency of Oxygen = 2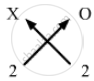
Formula for oxide of element X can be worked out by crossing the valencies of the constituent elements.
Therefore, formula for oxide of X is XO.
APPEARS IN
RELATED QUESTIONS
Write the symbols and valencies of the following radicals:
- Magnesium ion
- Ammonium
- Carbonate
- Nitrate
- Oxide
- Bisulphate
- Aluminium ion
Name the following radicals :
HC03
Name the following radicals :
OH
Give example.
Trivalent basic radicals
Write the basic radicals and acidic radicals of the following and then write the chemical formulae of these compound.
Calcium silicate
Write the basic radicals and acidic radicals of the following and then write the chemical formulae of these compound.
Potassium ferrocyanide
Write the basic radicals and acidic radicals of the following and then write the chemical formulae of these compound.
Sodium thiosulphate
State if the following element or radical is divalent?
Sulphite
State if the following element or radical is divalent?
Carbide
Atoms that carry positive or negative charges are called ______.
