Advertisements
Advertisements
Question
Balance the following reaction by oxidation number method.
\[\ce{Cr2O^2-_{7(aq)} + SO^2-_{3(aq)}->Cr^3+_{ (aq)} + SO^2-_{4(aq)}(acidic)}\]
Solution
\[\ce{Cr2O^2-_{7(aq)} + SO^2-_{3(aq)}->Cr^3+_{ (aq)} + SO^2-_{4(aq)}(acidic)}\]
Step 1: Write the skeletal equation and balance the elements other than O and H.
\[\ce{Cr2O^2-_{7(aq)} + SO^2-_{3(aq)}->2Cr^3+_{ (aq)} + SO^2-_{4(aq)}}\]
Step 2: Assign oxidation number to Cr and S. Calculate the increase and decrease in the oxidation number and make them equal.
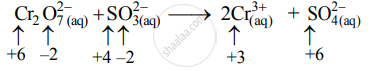
Increase in oxidation number:
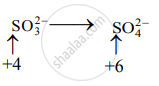
(Increase per atom = 2)
Decrease in oxidation number:
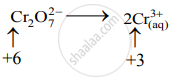
(Decrease per atom = 3)
To make the net increase and decrease equal, we must take 3 atoms of S and 2 atoms of Cr. (There are already 2Cr atoms.)
\[\ce{Cr2O^2-_{7(aq)} + 3SO^2-_{3(aq)}->2Cr^3+_{ (aq)} + 3SO^2-_{4(aq)}}\]
Step 3: Balance 'O' atoms by adding 4H2O to the right-hand side.
\[\ce{Cr2O^2-_{7(aq)} + 3SO^2-_{3(aq)}->2Cr^3+_{ (aq)} + 3SO^2-_{4(aq)} + 4H2O_{(l)}}\]
Step 4: The medium is acidic. To make the charges and hydrogen atoms on the two sides equal, add 8H+ on the left-hand side.
\[\ce{Cr2O^2-_{7(aq)} + 3SO^2-_{3(aq)} + 8H^+_{ (aq)}->2Cr^3+_{ (aq)} + 3SO^2-_{4(aq)} + 4H2O_{(l)}}\]
Step 5: Check two sides for the balance of atoms and charges.
Hence, balanced equation: \[\ce{Cr2O^2-_{7(aq)} + 3SO^2-_{3(aq)} + 8H^+_{ (aq)}->2Cr^3+_{ (aq)} + 3SO^2-_{4(aq)} + 4H2O_{(l)}}\]
APPEARS IN
RELATED QUESTIONS
Calculate the oxidation number of sulphur, chromium and nitrogen in H2SO5, `"Cr"_2"O"_7^(2-)` and `"NO"_3^-`. Suggest structure of these compounds. Count for the fallacy.
Balance the following redox reactions by ion-electron method:
- \[\ce{MnO-_4 (aq) + I– (aq) → MnO2 (s) + I2(s) (in basic medium)}\]
- \[\ce{MnO-_4 (aq) + SO2 (g) → Mn^{2+} (aq) + HSO-_4 (aq) (in acidic solution)}\]
- \[\ce{H2O2 (aq) + Fe^{2+} (aq) → Fe^{3+} (aq) + H2O (l) (in acidic solution)}\]
- \[\ce{Cr_2O^{2-}_7 + SO2(g) → Cr^{3+} (aq) + SO^{2-}_4 (aq) (in acidic solution)}\]
Balance the following equation in basic medium by ion-electron method and oxidation number methods and identify the oxidising agent and the reducing agent.
\[\ce{Cl_2O_{7(g)} + H_2O_{2(aq)} -> ClO-_{2(aq)} + O_{2(g)} + H+_{(aq)}}\]
Chlorine is used to purify drinking water. Excess of chlorine is harmful. The excess of chlorine is removed by treating with sulphur dioxide. Present a balanced equation for this redox change taking place in water.
Choose the correct option.
For the following redox reactions, find the correct statement.
\[\ce{Sn^{2⊕} + 2Fe^{3⊕}->Sn^{4⊕} + 2Fe^{2⊕}}\]
Justify that the following reaction is redox reaction; identify the species oxidized/reduced, which acts as an oxidant and which acts as a reductant.
\[\ce{2Cu2O_{(S)} + Cu2S_{(S)}->6Cu_{(S)} + SO2_{(g)}}\]
Balance the following reaction by oxidation number method.
\[\ce{MnO^-_{4(aq)} + Br^-_{ (aq)}->MnO2_{ (s)} + BrO^-_{3(aq)}(basic)}\]
Balance the following reaction by oxidation number method.
\[\ce{Bi(OH)_{3(s)} + Sn(OH)^-_{3(aq)}->Bi_{(s)} + Sn(OH)^2-_{6(aq)}(basic)}\]
Which of the following is INCORRECT for the following reaction?
\[\ce{2Zn_{(s)} + O2_{(g)} -> 2ZnO_{(s)}}\]
What is the change in oxidation number of Sulphur in following reaction?
\[\ce{MnO^-_{4(aq)} + SO^{2-}_{3(aq)} -> MnO^{2-}_{4(aq)} + SO^{2-}_{4(aq)}}\]
When methane is burnt completely, oxidation state of carbon changes from ______.
Write balanced chemical equation for the following reactions:
Reaction of liquid hydrazine \[\ce{(N2H4)}\] with chlorate ion \[\ce{(ClO^{-}3)}\] in basic medium produces nitric oxide gas and chloride ion in gaseous state.
Balance the following equations by the oxidation number method.
\[\ce{Fe^{2+} + H^{+} + Cr2O^{2-}7 -> Cr^{3+} + Fe^{3+} + H2O}\]
Balance the following equations by the oxidation number method.
\[\ce{I2 + NO^{-}3 -> NO2 + IO^{-}3}\]
Identify the redox reactions out of the following reactions and identify the oxidising and reducing agents in them.
\[\ce{3HCl (aq) + HNO3 (aq) -> Cl2 (g) + NOCl (g) + 2H2O (l)}\]
Identify the redox reactions out of the following reactions and identify the oxidising and reducing agents in them.
\[\ce{HgCl2 (aq) + 2KI (aq) -> HgI2 (s) + 2KCl (aq)}\]
Identify the redox reactions out of the following reactions and identify the oxidising and reducing agents in them.
\[\ce{Fe2O3 (s) + 3CO (g) ->[Δ] 2Fe (s) + 3CO2 (g)}\]
Identify the redox reactions out of the following reactions and identify the oxidising and reducing agents in them.
\[\ce{PCl3 (l) + 3H2O (l) -> 3HCl (aq) + H3PO3 (aq)}\]
In acidic medium, reaction, \[\ce{MNO^-_4 → Mn^2+}\] an example of ____________.
The weight of CO is required to form Re2(CO)10 will be ______ g, from 2.50 g of Re2O7 according to given reaction
\[\ce{Re2O7 + CO -> Re2(CO)10 + CO2}\]
Atomic weight of Re = 186.2; C = 12 and O = 16.
In the reaction of oxalate with permanganate in an acidic medium, the number of electrons involved in producing one molecule of CO2 is ______.
\[\ce{H2O2 -> 2H^+ + O2 + 2e^-}\]; E0 = −0.68 V.
This equation represents which of the following behaviour of H2O2?
