Advertisements
Advertisements
Question
Calculate the oxidation number of sulphur, chromium and nitrogen in H2SO5, `"Cr"_2"O"_7^(2-)` and `"NO"_3^-`. Suggest structure of these compounds. Count for the fallacy.
Solution
1)
+1 x -2
\[\ce{H2SO5}\]
2(+1) + 1(x) + 5(-2) = 0
⇒ 2 + x - 10 = 0
⇒ x = + 8
However, the O.N. of S cannot be +8. S has six valence electrons. Therefore, the O.N. of S cannot be more than +6.
The structure of H2SO5 is shown as follows:
\[\begin{array}{cc}
\ce{^{-2}O}\phantom{...........}\\
||\phantom{.........}\\
\ce{^{+1}H - ^{-2}O - S^{\text{x}} - ^{-1}O - ^{-1}O - ^{+1}H}\\
||\phantom{.........}\\
\ce{^{-2}O}\phantom{............}\end{array}\]
Now, 2(+1) + 1(x) + 3(-2) + 2(-1) = 0
⇒ 2 + x - 6 - 2 = 0
⇒ x = +6
Therefore, the O.N. of S is +6.
2)
x 2-
\[\ce{Cr2 O^{2-}_7}\]
2(x) + 7(-2) = -2
⇒ 2x - 14 = -2
⇒ x = +6
Here, there is no fallacy about the O.N. of Cr in `"Cr"_2"O"_7^(2-)`.
The structure of `"Cr"_2"O"_7^(2-)` is shown as follows:
\[\begin{array}{cc}
\ce{^{2-}O}\phantom{...........}\ce{^{2-}O}\phantom{....}\\
||\phantom{..............}||\phantom{..}\\
\ce{^{2-}O = Cr^{+6} - ^{2-}O - Cr^{+6} - O^{2-}}\\
|\phantom{..............}|\phantom{...}\\
\ce{_{1-}O^-}\phantom{.........}\ce{_{1-}O^-}\phantom{....}\end{array}\]
Here, each of the two Cr atoms exhibits the O.N. of +6.
3)
x 2-
\[\ce{N O^-_3}\]
1(x) + 3(-2) = -1
⇒ x - 6 = -1
⇒ x = +5
Here, there is no fallacy about the O.N. of N in `"NO"_3^(-)`
The structure of `"NO"_3^(-)` is shown as follows
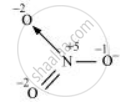
The N atom exhibits the O.N. of +5.
APPEARS IN
RELATED QUESTIONS
Consider the reaction:
\[\ce{O3(g) + H2O2(l) → H2O(l) + 2O2(g)}\]
Why it is more appropriate to write these reaction as:
\[\ce{O3(g) + H2O2 (l) → H2O(l) + O2(g) + O2(g)}\]
Also, suggest a technique to investigate the path of the redox reactions.
The compound AgF2 is an unstable compound. However, if formed, the compound acts as a very strong oxidizing agent. Why?
Whenever a reaction between an oxidising agent and a reducing agent is carried out, a compound of lower oxidation state is formed if the reducing agent is in excess and a compound of higher oxidation state is formed if the oxidising agent is in excess. Justify this statement giving three illustrations.
Balance the following redox reactions by ion-electron method:
- \[\ce{MnO-_4 (aq) + I– (aq) → MnO2 (s) + I2(s) (in basic medium)}\]
- \[\ce{MnO-_4 (aq) + SO2 (g) → Mn^{2+} (aq) + HSO-_4 (aq) (in acidic solution)}\]
- \[\ce{H2O2 (aq) + Fe^{2+} (aq) → Fe^{3+} (aq) + H2O (l) (in acidic solution)}\]
- \[\ce{Cr_2O^{2-}_7 + SO2(g) → Cr^{3+} (aq) + SO^{2-}_4 (aq) (in acidic solution)}\]
Balance the following equation in basic medium by ion-electron method and oxidation number methods and identify the oxidising agent and the reducing agent.
\[\ce{Cl_2O_{7(g)} + H_2O_{2(aq)} -> ClO-_{2(aq)} + O_{2(g)} + H+_{(aq)}}\]
The Mn3+ ion is unstable in solution and undergoes disproportionation to give Mn2+, MnO2, and H+ ion. Write a balanced ionic equation for the reaction.
Balance the following reaction by oxidation number method.
\[\ce{H2SO4_{(aq)} + C_{(s)}->CO2_{(g)} + SO2_{(g)} + H2O_{(l)}(acidic)}\]
Balance the following redox equation by half-reaction method.
\[\ce{Bi(OH)_{3(s)} + SnO^2-_{2(aq)}->SnO^2-_{3(aq)} + Bi^_{(s)}(basic)}\]
Which of the following is INCORRECT for the following reaction?
\[\ce{2Zn_{(s)} + O2_{(g)} -> 2ZnO_{(s)}}\]
Identify coefficients 'x' and 'y' for the following reaction.
\[\ce{{x}H2O2_{(aq)} + ClO^-_{4(aq)} -> 2O2_{(g)} + ClO^-_{2(aq)} + {y}H2O_{(l)}}\]
Which of the following is a redox reaction?
Write balanced chemical equation for the following reactions:
Reaction of liquid hydrazine \[\ce{(N2H4)}\] with chlorate ion \[\ce{(ClO^{-}3)}\] in basic medium produces nitric oxide gas and chloride ion in gaseous state.
Balance the following equations by the oxidation number method.
\[\ce{I2 + NO^{-}3 -> NO2 + IO^{-}3}\]
Balance the following equations by the oxidation number method.
\[\ce{I2 + S2O^{2-}3 -> I- + S4O^{2-}6}\]
Identify the redox reactions out of the following reactions and identify the oxidising and reducing agents in them.
\[\ce{PCl3 (l) + 3H2O (l) -> 3HCl (aq) + H3PO3 (aq)}\]
Balance the following ionic equations.
\[\ce{Cr2O^{2-}7 + Fe^{2+} + H+ -> Cr^{3+} + Fe^{3+} + H2O}\]
In \[\ce{Cu^{2+} + Ag -> Cu + Ag^+}\], oxidation half-reaction is:
The weight of CO is required to form Re2(CO)10 will be ______ g, from 2.50 g of Re2O7 according to given reaction
\[\ce{Re2O7 + CO -> Re2(CO)10 + CO2}\]
Atomic weight of Re = 186.2; C = 12 and O = 16.
\[\ce{H2O2 -> 2H^+ + O2 + 2e^-}\]; E0 = −0.68 V.
This equation represents which of the following behaviour of H2O2?
