Advertisements
Advertisements
Question
Balance the following reaction by oxidation number method.
\[\ce{Bi(OH)_{3(s)} + Sn(OH)^-_{3(aq)}->Bi_{(s)} + Sn(OH)^2-_{6(aq)}(basic)}\]
Solution
\[\ce{Bi(OH)_{3(s)} + Sn(OH)^-_{3(aq)}->Bi_{(s)} + Sn(OH)^2-_{6(aq)}(basic)}\]
Step 1: Write the skeletal equation and balance the elements other than O and H.
\[\ce{Bi(OH)_{3(s)} + Sn(OH)^-_{3(aq)}->Bi_{(s)} + Sn(OH)^2-_{6(aq)}}\]
Step 2: Assign oxidation numbers to Bi and Sn. Calculate the increase and decrease in the oxidation number and make them equal.
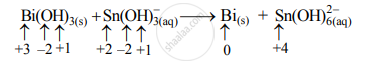
Increase in oxidation number:
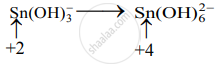
(Increase per atom = 2)
Decrease in oxidation number:

(Decrease per atom = 3)
To make the net increase and decrease equal, we must take 3 atoms of Sn and 2 atoms of Bi.
\[\ce{2Bi(OH)_{3(s)} + 3Sn(OH)^-_{3(aq)}->2Bi_{(s)} + 3Sn(OH)^2-_{6(aq)}}\]
Step 3: Balance ‘O’ atoms by adding 3H2O to the left-hand side.
\[\ce{2Bi(OH)_{3(s)} + 3Sn(OH)^-_{3(aq)} + 3H2O_{(l)}->2Bi_{(s)} + 3Sn(OH)^2-_{6(aq)}}\]
Step 4: The medium is basic. To make hydrogen atoms on the two sides equal, add 3H+ on the right-hand side.
\[\ce{2Bi(OH)_{3(s)} + 3Sn(OH)^-_{3(aq)} + 3H2O_{(l)}->2Bi_{(s)} + 3Sn(OH)^2-_{6(aq)} + 3H^+_{( aq)}}\]
Add OH− ions equal to the number of H+ ions on both sides of the equation.
\[\ce{2Bi(OH)_{3(s)} + 3Sn(OH)^-_{3(aq)} + 3H2O_{(l)} + 3OH^-_{( aq)}->2Bi_{(s)} + 3Sn(OH)^2-_{6(aq)} + 3H^+_{( aq)} + 3OH^-_{( aq)}}\]
The H+ and OH− ions appearing on the same side of the reaction are combined to give H2O molecules.
\[\ce{2Bi(OH)_{3(s)} + 3Sn(OH)^-_{3(aq)} + 3H2O_{(l)} + 3OH^-_{( aq)}->4Bi_{(s)} + 3Sn(OH)^2-_{6(aq)} + 3H2O_{(l)}}\]
\[\ce{2Bi(OH)_{3(s)} + 3Sn(OH)^-_{3(aq)} + 3OH^-_{( aq)}->2Bi_{(s)} + 3Sn(OH)^2-_{6(aq)}}\]
Step 5: Check two sides for balance of atoms and charges.
Hence, balanced equation: \[\ce{2Bi(OH)_{3(s)} + 3Sn(OH)^-_{3(aq)} + 3OH^-_{( aq)}->2Bi_{(s)} + 3Sn(OH)^2-_{6(aq)}}\]
APPEARS IN
RELATED QUESTIONS
Consider the reaction:
\[\ce{O3(g) + H2O2(l) → H2O(l) + 2O2(g)}\]
Why it is more appropriate to write these reaction as:
\[\ce{O3(g) + H2O2 (l) → H2O(l) + O2(g) + O2(g)}\]
Also, suggest a technique to investigate the path of the redox reactions.
The compound AgF2 is an unstable compound. However, if formed, the compound acts as a very strong oxidizing agent. Why?
Balance the following redox reactions by ion-electron method:
- \[\ce{MnO-_4 (aq) + I– (aq) → MnO2 (s) + I2(s) (in basic medium)}\]
- \[\ce{MnO-_4 (aq) + SO2 (g) → Mn^{2+} (aq) + HSO-_4 (aq) (in acidic solution)}\]
- \[\ce{H2O2 (aq) + Fe^{2+} (aq) → Fe^{3+} (aq) + H2O (l) (in acidic solution)}\]
- \[\ce{Cr_2O^{2-}_7 + SO2(g) → Cr^{3+} (aq) + SO^{2-}_4 (aq) (in acidic solution)}\]
Balance the following equation in the basic medium by ion-electron method and oxidation number methods and identify the oxidising agent and the reducing agent.
\[\ce{N2H4(l) + ClO^-_3 (aq) → NO(g) + Cl–(g)}\]
The Mn3+ ion is unstable in solution and undergoes disproportionation to give Mn2+, MnO2, and H+ ion. Write a balanced ionic equation for the reaction.
Chlorine is used to purify drinking water. Excess of chlorine is harmful. The excess of chlorine is removed by treating with sulphur dioxide. Present a balanced equation for this redox change taking place in water.
Choose the correct option.
For the following redox reactions, find the correct statement.
\[\ce{Sn^{2⊕} + 2Fe^{3⊕}->Sn^{4⊕} + 2Fe^{2⊕}}\]
Balance the following reaction by oxidation number method.
\[\ce{Cr2O^2-_{7(aq)} + SO^2-_{3(aq)}->Cr^3+_{ (aq)} + SO^2-_{4(aq)}(acidic)}\]
Balance the following redox equation by half-reaction method.
\[\ce{H2C2O_{4(aq)} + MnO^-_{4(aq)}->CO2_{(g)} + Mn^2+_{( aq)}(acidic)}\]
Balance the following redox equation by half-reaction method.
\[\ce{Bi(OH)_{3(s)} + SnO^2-_{2(aq)}->SnO^2-_{3(aq)} + Bi^_{(s)}(basic)}\]
Identify the oxidising agent in the following reaction:
\[\ce{CH4_{(g)} + 2O2_{(g)} -> CO2_{(g)} + 2H2O_{(l)}}\]
When methane is burnt completely, oxidation state of carbon changes from ______.
Consider the reaction:
\[\ce{6 CO2(g) + 6H2O(l) → C6 H12O6(aq) + 6O2(g)}\]
Why it is more appropriate to write these reaction as:
\[\ce{6CO2(g) + 12H2O(l) → C6 H12O6(aq) + 6H2O(l) + 6O2(g)}\]
Also, suggest a technique to investigate the path of the redox reactions.
Write balanced chemical equation for the following reactions:
Permanganate ion \[\ce{(MnO^{-}4)}\] reacts with sulphur dioxide gas in acidic medium to produce \[\ce{Mn^{2+}}\] and hydrogen sulphate ion.
Write balanced chemical equation for the following reactions:
Reaction of liquid hydrazine \[\ce{(N2H4)}\] with chlorate ion \[\ce{(ClO^{-}3)}\] in basic medium produces nitric oxide gas and chloride ion in gaseous state.
Balance the following equations by the oxidation number method.
\[\ce{Fe^{2+} + H^{+} + Cr2O^{2-}7 -> Cr^{3+} + Fe^{3+} + H2O}\]
Balance the following equations by the oxidation number method.
\[\ce{MnO2 + C2O^{2-}4 -> Mn^{2+} + CO2}\]
Identify the redox reactions out of the following reactions and identify the oxidising and reducing agents in them.
\[\ce{3HCl (aq) + HNO3 (aq) -> Cl2 (g) + NOCl (g) + 2H2O (l)}\]
Identify the redox reactions out of the following reactions and identify the oxidising and reducing agents in them.
\[\ce{HgCl2 (aq) + 2KI (aq) -> HgI2 (s) + 2KCl (aq)}\]
Identify the redox reactions out of the following reactions and identify the oxidising and reducing agents in them.
\[\ce{PCl3 (l) + 3H2O (l) -> 3HCl (aq) + H3PO3 (aq)}\]
Identify the redox reactions out of the following reactions and identify the oxidising and reducing agents in them.
\[\ce{4NH3 (g) + 3O2 (g) -> 2N2 (g) + 6H2O (g)}\]
In the reaction of oxalate with permanganate in an acidic medium, the number of electrons involved in producing one molecule of CO2 is ______.
\[\ce{H2O2 -> 2H^+ + O2 + 2e^-}\]; E0 = −0.68 V.
This equation represents which of the following behaviour of H2O2?
