Advertisements
Advertisements
Question
Compounds such as alcohol and glucose also contain hydrogen but are not categorised as acids. Describe an activity to prove it.
Solution
Activity to show that alcohol and glucose also contain hydrogen but are not acids:
Procedure:
i) Take samples of alcohol, glucose and hydrochloric acid.
ii) Take a 100 ml beaker. Take a cork and fix two nails on it.
iii) Place the cork inside the beaker as shown in the figure.
iv) Connect the nails to two terminals of a 6-volt battery through a bulb and a switch.
v) Now pour some dilute hydrochloric acid in the beaker so that the nails and the cork are immersed in it.
vi) Switch on the current flow.
vii) Repeat the same with alcohol and glucose.
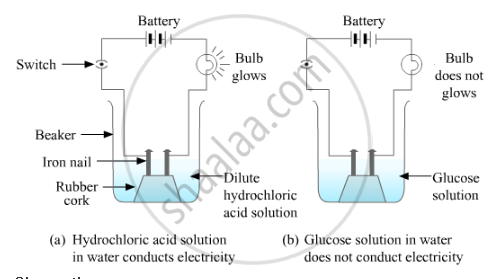
Observation :
The bulb starts glowing in the case of acid, whereas it does not glow in the case of glucose and alcohol solutions. Glowing of the bulb signifies that there is a flow of electric current through the solution. The electric current is carried through the solution by ions.
Conclusion:
Acids dissociate in aqueous solutions to give H+(aq) ions, which determine their acidic property. Glucose and alcohol do not dissociate and do not furnish H+ ions in aqueous solutions even though they contain hydrogen atoms. Hence, HCl shows acidic character in aqueous solutions, whereas solutions of compounds such as C6H12O6 (glucose) and C2H5OH (alcohol) do not show acidic character.
APPEARS IN
RELATED QUESTIONS
Why do HCl, HNO3, etc., show acidic characters in aqueous solutions while solutions of compounds like alcohol and glucose do not show acidic character?
Complete and balance the followingchemicalequations:
NaHCO3 (S) + HCI (aq) →
Fill in the blank in the following sentences:
Substances do not show their acidic properties without.......................... .
On adding dilute hydrochloric acid to copper oxide powder, the solution formed is blue-green.
On the basis of the above reaction, what can you say about the nature of copper oxide?
A substance X which is used as an antacid reacts with dilute hydrochloric acid to produce a gas Y which is used in one type of fire-extinguisher. Name the substance X and gas Y. Write a balanced equation for the chemical reaction which takes place.
What is the chemical formula of washing soda?
Common salt besides being used in kitchen can also be used as the raw material for making
- washing soda
- bleaching powder
- baking soda
- slaked lime
Name the acid present in ant sting and give its chemical formula. Also give the common method to get relief from the discomfort caused by the ant sting.
______ change the colour of the indicators.
Which acid is present in milk?
