Advertisements
Advertisements
Question
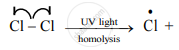
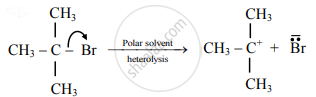
Distinguish between Homolysis and heterolysis.
Solution
| No. | Homolysis (Homolytic fission) | Heterolysis (Heterolytic fission) |
| 1. | The symmetrical breaking of a covalent bond in which each departing atom takes one electron from the bonding pair is called as homolytic fission. | The unsymmetrical breaking of a covalent bond in which one of the departing atoms retains the bonding pair is called heterolytic fission. |
| 2. | In this type of fission, the formation of free radicals (uncharged species) bearing unpaired electrons takes place. | In this type of fission, the formation of charged species called ions, like carbocation or carbonium ion takes place. |
| 3. |
The covalent bond between two atoms of the same element or two atoms having nearly the same electronegativity breaks in this manner.
|
The covalent bond between two atoms of the different elements or two atoms having different electronegativity values breaks in this manner.
|
| 4. | This takes place favourably in a nonpolar solvent. | This takes place favourably in a polar solvent. |
| 5. | Generally, reaction takes place at high temperature or in presence of UV light or peroxides. | Heterolysis takes place in solutions (polar condition). |
APPEARS IN
RELATED QUESTIONS
Find out the most stable species from the following. Justify.
`bar"C""H"_3, bar"C""H"_2"Br", bar"C""Br"_3`
Find out the most stable species from the following. Justify.
\[\ce{\overset{+}{C}H3, \overset{+}{C}H2Cl, \overset{+}{C}Cl3}\]
Identify the α - carbons in the following species and give the total number of α-hydrogen.
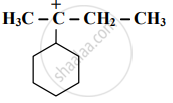
Identify the α-carbon in the following species and give the total number of α-hydrogens.
\[\ce{CH2 = CH - CH2 - CH3}\]
Draw a resonance structure of the following:
Phenol
Draw a resonance structure of the following:
Acetate ion
Write true or false. Correct the false statement.
Homolytic fission involves the unsymmetrical breaking of a covalent bond.
Choose the correct option.
Hyperconjugation involves overlap of ______ orbitals.
Choose the correct option.
The geometry of a carbocation is ______.
Which of the following is NOT an electrophile?
Which among the following is a set of nucleophiles?
Which of the following is TRUE for homolytic fission?
The best reagent for the following conversion is:
The +I inductive effect is shown by which of the following groups?
Which of the following alkyl groups shows maximum positive inductive effect?
The overlap of σ-p orbitals is called ____________.
Which of the following is the most unstable carbocation?
IUPAC name of ![]() is ______.
is ______.
Which of the following alkyl groups shows least positive inductive effect?
How many tertiary carbon atoms and primary carbon atoms respectively are present in 2-iodo-3, 3- dimethyl pentane?
How many pi bonds and sigma bonds are present in following molecule?
Which element among the following does form pπ - pπ multiple bonds?
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH2 = CH - CH2 - CH3}\]
Identify the α - carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
Identify the α - carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
Identify the α-carbons in the following species and give the total number of α-hydrogen in it.
CH2 = CH − CH2 − CH3
Identify the α-carbon in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
Identify the α-carbons in the following species and give the total number of α-hydrogens.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]