Advertisements
Advertisements
Question
Answer the following question.
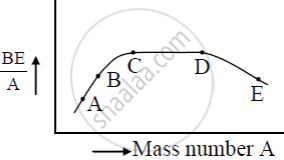
Draw the curve showing the variation of binding energy per nucleon with the mass number of nuclei. Using it explains the fusion of nuclei lying on the ascending part and fission of nuclei lying on the descending part of this curve.
Solution

The above curve tells us that the binding energy per nucleon is smaller for heavier nuclei as well as for lighter nuclei than for the middle order nuclei (with mass number lying between 30 to 170). Meaning heavier nuclei are less stable thus they undergo fission and lighter nuclei undergo fusion in order to form the nucleus lying in the range of the mass number 30 to 170.
APPEARS IN
RELATED QUESTIONS
Is the nucleus formed in the decay of the nucleus `""_11^22Na`, an isotope or isobar?
If the nucleons of a nucleus are separated from each other, the total mass is increased. Where does this mass come from?
In which of the following decays the atomic number decreases?
(a) α-decay
(b) β+-decay
(c) β−-decay
(d) γ-decay
The figure shows the plot of binding energy (BE) per nucleon as a function of mass number A. The letters A, B, C, D, and E represent the positions of typical nuclei on the curve. Point out, giving reasons, the two processes (in terms of A, B, C, D, and E ), one of which can occur due to nuclear fission and the other due to nuclear fusion.
The difference in mass of a nucleus and its constituents is called ______.
Mx and My denote the atomic masses of the parent and the daughter nuclei respectively in a radioactive decay. The Q-value for a β– decay is Q1 and that for a β+ decay is Q2. If m e denotes the mass of an electron, then which of the following statements is correct?
The deuteron is bound by nuclear forces just as H-atom is made up of p and e bound by electrostatic forces. If we consider the force between neutron and proton in deuteron as given in the form of a Coulomb potential but with an effective charge e′: F = `1/(4πε_0) e^('2)/r` estimate the value of (e’/e) given that the binding energy of a deuteron is 2.2 MeV.
Find the binding energy of a H-atom in the state n = 2
Which of the following quantities is a measure of stability of nucleus?
What is meant by “binding energy per nucleon” of a nucleus?
